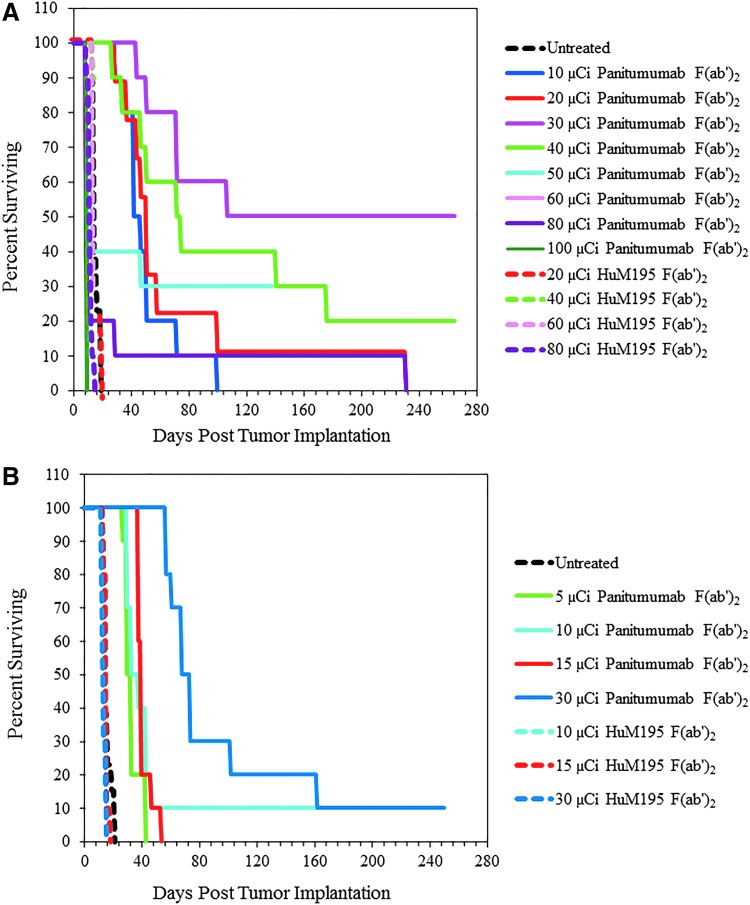
FIG. 4.
A dose escalation study was performed with 212Pb-panitumumab F(ab′)2 to determine an effective therapeutic dose delivered either by an i.p. or i.v. route. For the i.p. injection (A), groups of athymic mice (n = 10) bearing 3D LS-174T i.p. tumor xenografts were injected with 10, 20, 30, 40, 50, 60, 80, or 100 μCi of 212Pb-panitumumab F(ab′)2. Additional groups of mice were administered 20, 40, 60, or 80 μCi of 212Pb-HuM195 F(ab′)2, which served as a nonspecific control. The mice receiving the 212Pb-RIT by i.v. injection (B) were injected with 5, 10, 15, or 30 μCi of 212Pb-panitumumab F(ab′)2 or 10, 15, or 30 μCi of 212Pb-HuM195. An eighth cohort of mice was left untreated.