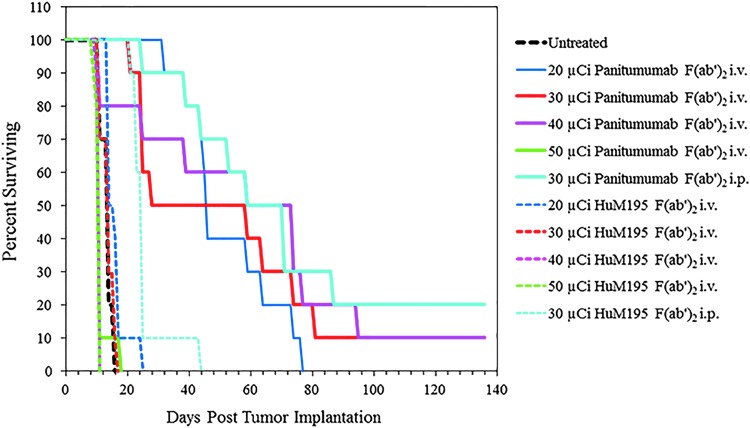
FIG. 5.
A dose escalation study was repeated at higher doses to establish the effective, suboptimal, therapeutic dose for subsequent studies with chemotherapeutics. Athymic mice (n = 10) bearing 3 d i.p. LS-174T tumor xenografts were injected i.v. with 20, 30, 40, and 50 μCi of 212Pb-panitumumab F(ab′)2. Additional groups included those that received no treatment and those that were injected with the nonspecific control, 212Pb-HuM195 F(ab′)2 at the same dosing level as the 212Pb-panitumumab F(ab′)2. The study also included cohorts of mice that were injected i.p. with 30 μCi of the each of the 212Pb-labeled F(ab′)2 fragments to validate the i.p. dosage.