Figure 4.
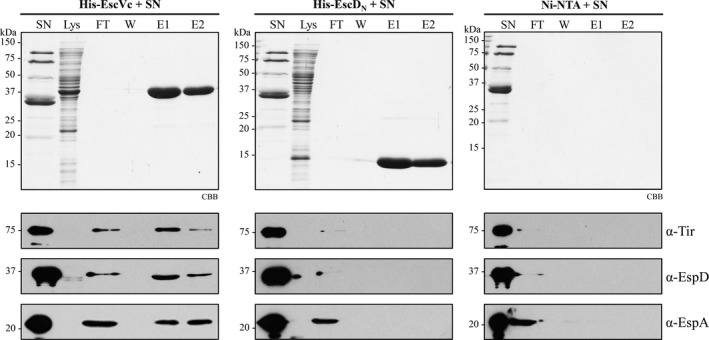
EscVc directly interacts with secreted proteins. Pull‐down assay of His‐EscVc (left panels) and His‐EscDN (middle panels) with secreted proteins, performed by nickel affinity chromatography. The cleared lysate containing His‐EscVc or His‐EscDN (Lys), was incubated with Ni‐NTA beads and loaded into a column. The proteins secreted into the supernatant by the ∆sepL and ∆grlR null mutant strains (SN) were passed through the His‐EscVc or His‐EscDN‐coupled resin in the column and the flow through (FT) was collected. After extensive washing (W), proteins were eluted (E1 and E2). Samples were visualized by SDS‐PAGE stained with Coomassie brilliant blue (CBB) (upper panels). Detection of copurified proteins was performed by immunoblotting with specific antibodies against Tir, EspD, and EspA (lower panels). As an additional control, the nonspecific binding of Tir, EspD, and EspA to the Ni‐NTA beads was analyzed by SDS‐PAGE stained with CBB and immunoblotting (right panels)
