Figure 6.
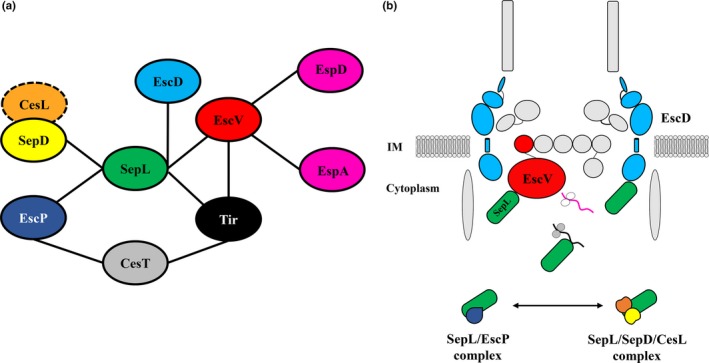
SepL protein–protein interaction network involved in middle and late substrate secretion regulation. (a) The SepL protein binds directly to five different T3SS components: SepD, EscP, Tir, EscD, and EscV. Although SepL forms a ternary complex with SepD and CesL (Younis et al., 2010), no direct interaction between CesL (dashed circle) and SepL or SepD has been reported. The effector Tir binds to its cognate chaperone CesT (Elliott et al., 1999), which in turn has been demonstrated to interact with EscP (Monjaras Feria et al., 2012). Finally, the interaction between EscV and different T3S substrates is also displayed. (b) Schematic representation of the EPEC T3SS base showing the SepL binding partners described so far. SepL [green] interacts with EscD [cyan], Tir [black] (Wang et al., 2008), and EscV [red] (this work). In addition, SepL was reported to form two mutually exclusive complexes: the SepL‐EscP [blue] complex, which was proposed to regulate substrate secretion in response to calcium changes in vitro (Shaulov et al., 2017); and the SepL‐SepD [yellow]‐CesL [orange] complex (Younis et al., 2010), whose role in substrate secretion regulation remains to be determined
