Abstract
STUDY QUESTION
Is allograft inflammatory factor-1 (AIF-1), a cytokine associated with inflammation and allograft rejection, aberrantly elevated in in vitro fertilization (IVF) cycles with gonadotropin-releasing hormone (GnRH) antagonist protocol with potential effects on endometrial receptivity?
SUMMARY ANSWER
Our findings indicated AIF-1 is increased in IVF cycles with GnRH antagonist protocol and mediates greater TNF-α expression during implantation phase, which may be unfavorable for embryo implantation.
WHAT IS KNOWN ALREADY
Studies have shown that GnRH antagonist protocol cycles have lower implantation and clinical pregnancy rates than GnRH agonist long protocol cycles. Endometrial receptivity but not embryo quality is a key factor contributing to this phenomenon; however, the mechanism is still unknown.
STUDY DESIGN, SIZE, DURATION
Implantation and pregnancy rates were studied in 238 patients undergoing their first cycle of IVF/ICSI between 2012 and 2014. Forty of these patients opted to have no fresh embryo replacement and were divided into two equal groups: (i) GnRH antagonist protocol and (ii) GnRH agonist long protocol, group 3 included 20 infertile women with a tubal factor in untreated cycles. During the same interval, endometrial tissues were taken from 18 infertile women with a tubal factor in the early proliferative phase, late proliferative phase, and mid-secretory phase of the menstrual cycle (n = 6/group).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Microarray analysis, RT-qPCR, Western blot analysis, immunohistochemistry were used to investigate the expression levels of AIF-1 and the related cytokines (TNF-α, IL1β, IL1RA, IL6, IL12, IL15 and IL18). The effect of AIF-1 on uterine receptivity was modeled using in vitro adhesion experiments (coculture of JAR cells and Ishikawa cells).
MAIN RESULTS AND THE ROLE OF CHANCE
The expression of AIF-1 was the highest in early proliferative phase, decreasing thereafter in the late proliferative phase, and almost disappearing in the mid-secretory phase, indicating that low AIF-1 expression might be important for embryo implantation during implantation phase. Microarray results revealed that AIF-1 was upregulated in the antagonist group compared with the control group (fold change [FC] = 3.75) and the agonist (FC = 2.20) group. The raw microarray data and complete gene expression table were uploaded to GEO under the accession number of GSE107914. Both the mRNA and protein expression levels of AIF-1 and TNF-α were the higher in the antagonist group than in the other two groups (P < 0.05) which did not differ significantly (P > 0.05). The protein levels of TNF-α in both Ishikawa cells and primary endometrial cells were significantly increased (P < 0.05) at 96 h after transfection with the AIF-1 expression vector, indicating that TNF-α was mediated by AIF-1 in endometrial cells. Overexpression of AIF-1 in Ishikawa cells inhibited adhesion of JAR cells to them. Thus, increased AIF-1 might inhibit adhesion during implantation via raised TNF-α.
LIMITATIONS REASONS FOR CAUTION
The sample size of the microarray was small, which might weaken the accuracy of our results; however, the sample size of RT-qPCR and the Western blotting assays were sufficient to compensate for this deficiency in our study. In addition, the aberrant AIF-1 and thus TNF-α expression is one of many factors that may contribute to limiting implantation success. Therefore, further extensive in vitro mechanistic and in vivo animal studies are needed to assess the actual functional impact of this pathway.
WIDER IMPLICATIONS OF THE FINDINGS
Anti-TNF-α therapy might mitigate the adverse effects of GnRH antagonist on endometrial receptivity and improve the implantation rate in GnRH antagonist protocols in IVF.
STUDY FUNDING/COMPETING INTERESTS
This work was supported by grants from the National Natural Science Foundation of China, Grant numbers 81771656 and 81370763; Clinical research special fund of Chinese Medical Association, Grant number 16020480664; Shanghai Jiao Tong University Medicine-Engineering Fund, Grant number YG2017ZD11 and YG2017MS57; and the Merck-Serono China Research Fund for Fertility Agreement. P.C.K.L. is supported by a Canadian Institutes of Health Research Foundation Scheme Grant 143317. None of the authors has any competing interests.
Keywords: AIF-1, TNF-α, endometrial receptivity, GnRH antagonist, implantation rate, IVF
Introduction
Gonadotropin-releasing hormone antagonists (GnRH-ant) in ARTs seemed to be one of the most ‘friendly’ IVF procedures because of several advantages, including short treatment duration, lower gonadotropin requirement, a lack of hypoestrogenism and a reduction in the incidence of severe ovarian hyperstimulation syndrome (Huirne et al., 2007; Al-Inany et al., 2016). However, a series of studies showed that GnRH-ant protocol cycles were associated with lower implantation and clinical pregnancy rates than GnRH agonist long protocol cycles (Al-Inany et al., 2007; Kdous et al., 2009; Lambalk et al., 2017). Our data also showed that both the implantation rate (24.8%) and pregnancy rate (32%) in GnRH-ant protocol group were lower than those in the agonist long group (37.7% and 51%, respectively) (Supplementary Table SI). Endometrial receptivity, and not embryo quality, might be the key factor which explains to this observation (Cota et al., 2012; Meng et al., 2014) but the effect of GnRH-ant treatment on the endometrium remains to be fully elucidated.
In our previous study, the numbers of uterine natural killer cell and perforin expression levels were both increased in endometrium from GnRH-ant-treated patients, suggesting that GnRH-ant might increase the release of proinflammatory factors during implantation phase (Xu et al., 2017). On the other hand, treatment with GnRH-ant has also been shown to alter the expression of immunomodulatory cytokines of the endometrium (Raga et al., 2008), which might affect embryo implantation. From an immunological perspective, maternal immune tolerance is essential for embryo implantation since the conceptus is considered a semiallograft to the maternal immune system. Allograft inflammatory factor-1 (AIF-1) is associated with inflammatory response and defense that is found in allograft tissues under chronic rejection (Shimada et al., 2003). In this study, we aimed to investigate the possible role of AIF-1 in reducing endometrial receptivity in GnRH-ant IVF protocol. Our results might provide theoretical support for the underlying mechanisms of this phenomenon and inform the future application of AIF-1 in ovarian stimulation in IVF.
Methods and materials
Patients
Between January 2012 and December 2014, a total of 238 women who received in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) for the first time at the Reproductive Medical Center of our hospital was included. All experiments were performed in accordance with the relevant guidelines and regulations. All the subjects signed informed consents, and the Institutional Review Board-approved protocol, which was approved by the Shanghai Jiaotong University Committee on the Use of Human Subjects in Medical Research. Further information including the stimulation protocol has been described previously (Xu et al., 2017). Final oocyte maturation was achieved by administration of 5000IU of hCG (Livzon, China). Details and clinical outcomes are shown in Supplementary Table SI. A total of 40 women, who opted out of fresh embryo transfer for personal reasons and elected to have all their embryos cryopreserved, was recruited in this study. They were divided into two groups (n = 20 per group): (i) GnRH antagonist protocol and (ii) GnRH agonist long protocol. Endometrial specimens were obtained from pipe suction curettage (Wallace) on Day 7 after oocyte recovery and from 20 infertile women with tubal factor in untreated cycles on day LH + 7 as controls. In the meantime, 18 infertile women, with tubal factor without any treatment in natural cycle who received hysteroscopy, were further divided into three groups (n = 6 per group): (i) early proliferative phase (days 4–5 of the cycle), (ii) late proliferative phase (days 11–13 of the cycle) and (iii) mid-secretory phase (days 20–22 of cycle). The inclusion and exclusion criteria were the same as previous 60 patients (Xu et al., 2017). Some endometrial tissues were obtained from each person by hysteroscopy. All specimens were frozen at –80°C until subsequent analysis.
Microarray studies
Agilent human gene expression 8 × 60 K (Design ID:039494) arrays were used to examine the whole genome expression profiling of endometrial tissue from three individuals from each group as previously described (Xu et al., 2017). Data from GEO under the accession number of GSE107914 were analyzed and the differentially expressed genes were exhibited when FC ≥ 2 and adjusted P-value ≤0.05.
Primary epithelial and stromal cell isolation
Primary human endometrial epithelial and stromal cells were used as the study models. The isolation procedure was based on the previous protocol (Zhang et al., 2012) with slight modifications. Briefly, both primary cells were isolated from fresh endometrial biopsies obtained from six untreated women during days 20–22 of cycle. The tissues were minced finely and incubated at 37°C in a shaker for 45 min in 30 ml DMEM/F12 (Gibco, USA) containing 0.2% (w/v) collagenase (type I; Sigma, UK). Then the digest was filtered successively through 100-sieve mesh (150 μm) and 400-sieve mesh (37 μm). Stromal cells were collected by centrifuging the filtrate at 100 g for 5 min. Epithelial cells were collected by reverse flushing the 400-sieve mesh with DMEM/F12 medium and centrifugation at 100 g for 5 min. The isolated primary epithelial and stromal cells were plated on dishes (tissue culture-treated by vacuum gas plasma, Corning, USA, 3516) with complete medium containing DMEM/F12, 1% (v/v) penicillin/streptomycin (Gibco, USA) and 10% (v/v) fetal bovine serum (Gibco, USA) and were incubated in a 37°C incubator with humidified air which contains 5% CO2.
Cell culture and cell transfection
Both primary cells and Ishikawa cells were cultured in DMEM/F12 medium containing 1% (v/v) penicillin/streptomycin and 10% (v/v) fetal bovine serum, and JAR cells were cultured in RPMI-1640 medium. Additionally, the primary endometrial epithelial and stromal cells (about 5–7 cell masses per 100× magnification high power field) used were cultured separately in six-well plates after isolation and were used fresh without being subjected to a freeze-down and recover processes. All the cells were incubated in a 37°C incubator with 5% CO2. Cells were transfected with the AIF-1 expression plasmid DNA using the X-tremeGENE 9 DNA transfection reagent according to the manufacturer’s instructions (Roche, USA). Briefly, cells were plated in six-well plates for 24 h before the initiation of transfection (around 50% confluence). On the second day, AIF-1 expression plasmid (GeneCopoeia, USA) together with control (Puc19 plasmid) was transfected into cells with transfection reagent (1:3 ratio: DNA Vs reagent). Each transfection related experiment in this article was repeated at least thrice.
RNA isolation and RT-qPCR
To validate profiling data, total RNA was extracted from endometrial biopsy tissues using Trizol (Invitrogen) and reverse transcribed with a cDNA Reverse Transcription Kit (Toyobo, Japan). For subsequent research, RNA was extracted from endometrial biopsy tissues and Ishikawa cells at 0, 24, 48, 72 and 96 h after transfection with the AIF-1 expression plasmid, and samples were stored at −80°C before they were reverse transcribed into cDNA. cDNA synthesis was carried out using a PrimeScript RT Master Mix (Takara, RR036A) in accordance with the manufacturer’s instructions. The cDNA was stored at −20°C until used.
RT-qPCR was performed with SYBR Premix Ex Taq kit (Takara, RR420A). The sequences of the primer pairs used for RT-qPCR are listed in Table I. Each reaction included an initial denaturation step at 95°C for 30 min, followed by 40 cycles of amplification at 95°C for 5 s and annealing at 60°C for 34 s. Gene expression was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Fold change (FC) was calculated according to the 2–ΔΔCt method and a P-value of <0.05 was considered statistically significant. Each biopsy or cell sample was analyzed in triplicate.
Table I.
Genes and corresponding primers used for RT-qPCR.
| Gene product | Sense primer | Antisense primer |
|---|---|---|
| AIF-1 | AGACGATCCCAAATATAGCAG | TAGCTCTAGGTGAGTCTTGG |
| TNF-α | CTCCAGGCGGTGCTTGTTC | GGCTACAGGCTTGTCACTCG |
| IL1β | AATGATGGCTTATTACAGTGGCAATG | TAGTGGTGGTCGGAGATTCGTAG |
| IL1RA | CTAAGGTGGAGGATTCAGGACATTAC | CAACGGGTAGTTTCTGCTTAAATATGG |
| IL6 | AGAACAGATTTGAGAGTAGTGAGGAAC | GGCATTTGTGGTTGGGTCAGG |
| IL12 | CGCAGCCTCCTCCTTGTGG | ATGGGAACATTCCTGGGTCTGG |
| IL15 | TCCATCCAGTGCTACTTGTGTTTAC | CTTATTACATTCACCCAGTTGGCTTC |
| IL18 | CTCTTCATTGACCAAGGAAATCGG | TTCACAGAGATAGTTACAGCCATACC |
| GAPDH | TGACTTCAACAGCGACACCCA | CACCCTGTTGCTGTAGCCAAA |
Protein extraction and western blotting
Protein was extracted from endometrial tissues and transfected cells (96 h) with RIPA lysis buffer (Thermo, USA) containing 1% (v/v) protease inhibitor cocktail (Roche, Germany). Protein concentrations were quantified using the BCA protein assay kit (Beyotime, China). Samples with a total amount of 60 μg protein were separated by SDS-PAGE and transferred onto a 0.22-μm polyvinylidene difluoride (PVDF) membranes (Millipore, USA). Membranes were blocked in 5% (w/v) nonfat milk for 1 h at room temperature (For TNF-α, membranes were blocked with 1% (w/v) fetal bovine serum) and were incubated overnight at 4°C with specific primary antibodies against AIF-1 (1:200, Sangon biotech, China, D120798); TNF-α (1:1000, Abcam, USA, ab6671); IL-1β (1:1000, Abcam, USA, ab2105); IL1RA (1:5000, Abcam, USA, ab124962) and GAPDH (1:1000, Cell Signaling, USA, #5174). After washing thrice with Tris-buffered saline and 0.1% (v/v) Tween-20 (TBST), membranes were incubated with anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000, Cell Signaling, USA, #7074) for 1 h at room temperature. Membranes were washed with TBST and signals were detected using enhanced chemiluminescence (Millipore, USA), according to the manufacturers’ instructions.
Immunohistochemistry
Briefly, endometrial tissues from patients of three groups were fixed with formalin and then embedded in paraffin. Sections (7 μm) were deparaffinized in xylene and were rehydrated in a graded ethanol series to dewax. All the sections used were boiled in citrate buffer (10 mM; pH 6.0) for 20 min and then were incubated in a humidified chamber with trypsin (1:3; Abcam, USA, ab970) for 5 min for antigen retrieval. All sections to be stained for AIF-1 and TNF-α were immersed in 3% (v/v) hydrogen peroxide (in 50% methanol/50% distilled water, v/v) for 10 min to block endogenous peroxidase activity. After washing thrice in tris-buffered saline (TBS; pH 7.6), the sections were incubated in a humidified chamber with 5% (v/v) normal goat serum in TBS for 30 min at room temperature to block nonspecific binding. After removing excess serum, the slides were incubated with rabbit anti-AIF-1 polyclonal antibodies (1:200; Sangon biotech, China, D120798), rabbit anti-TNF-α polyclonal antibodies (1:100; Abcam, USA, ab6671), or normal rabbit IgG antibodies overnight at 4°C in a humidified chamber. Slides were then stained with secondary antibodies followed by hematoxylin counterstaining. The images were visualized using a microscope (Leica SP8, Germany).
To compare AIF-1 and TNF-α protein expression in the endometrium between groups, the intensity of protein expression was semiquantified by two pathologists in a blinded fashion with IHC-scores using the following rules: IHC-score = Σ (% of cells immunostained × average intensity of staining). Intensity of AIF-1 and TNF-α staining was divided into four bands (negative staining = 0, weak staining = 1, moderate staining = 2 and strong staining = 3). Areas (600 × 600 pixels) for examination were chosen at random from each 400× magnification high power field but were only scored if they showed a balance of epithelial and stromal cells; the average IHC-score of chosen areas for each section was used for analysis.
Immunocytochemistry
To verify the primary endometrial epithelial and stromal cells, they were stained for cytokeratin and vimentin, respectively. Cells seeded on coverslips in 24-well plates were fixed with 4% (w/v) paraformaldehyde, then permeabilized with 0.2% (v/v) Triton X-100 for 10 min at room temperature. After being blocked with 5% (w/v) bovine serum albumin (Amresco, USA), the epithelial and stromal cells were incubated with anticytokeratin (1:1000, Abcam, USA, ab181595) or antivimentin (1:500, Abcam, USA, ab92547) overnight at 4°C, respectively. After washing three times with PBS, antirabbit Alexa Fluor 488 (1:1000, Abcam, USA, ab150077) or antirabbit Alexa Fluor 594 (1:1000, Abcam, USA, ab150080) was incubated with the cells at 37°C for 1 h. After washing three times with PBS, the slips were stained with Hoechst34580 (Sigma-Aldrich, USA, 63493) to visualize nucleus. Images were captured using fluorescence microscope (Leica SP8, Germany).
Cell adhesion assay and lentivirus transduction
Ishikawa cells that were transduced with negative or AIF-1 overexpression lentivirus (gift from Dr. Demin Yu, Infectious Diseases department of Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China) were seeded into six-well plates. The packaging and transduction of AIF-1 overexpressed lentivirus were performed with Lentivector Expression System (System Biosciences, SBI, USA) according to the manufacturer’s instruction. Briefly, AIF-1 ORF was inserted into the lentiviral expression vector (with upstream of CMV promotor) followed by the transduction of this vector into 293T cells with packaging plasmids. Pseudoviral particles containing AIF-1 ORF and puromycin-resistant marker from supernatant were transduced into Ishikawa cells. Following 72 h post-transduction, the cells were selected by puromycin (0.1 μg/ml) for 4 days. This selection was repeated once more. Finally, the selected cells were cultured in medium containing 0.05 μg/ml puromycin. The adhesion assay was performed as previously described (HJ et al., 2017) with slight modification. The JAR cells were labeled with Calcein (Yeasen, China) for 30 min in a 37°C incubator before adding to confluent Ishikawa monolayers in RPMI-1640 medium. These cells were cocultured in a 37°C incubator for 1 h and subjected to vigorous shaking at 140 rpm for 5 min before refreshing medium. Finally, cells were imaged using a fluorescent microscope (Leica V3.8). The experiment was repeated thrice.
Statistical analysis
Results are expressed as mean ± SEM. Differences between two groups were analyzed using independent sample t-test. Differences between more than two groups were analyzed using one-way analysis of variance if normality (and homogeneity of variance) assumptions are satisfied and Bonferroni test was used to analyze multiple comparisons. Otherwise, the Kruskal–Wallis test followed by Mann–Whitney U-test was used to analyze the data. All statistical analyses were performed using the SPSS 22.0 (IBM, Inc.) and statistical significance was defined as P < 0.05.
Results
Demographic characteristics
There was no significant difference among the recruited three groups regarding the demographic characteristics, as we previously described (Xu et al., 2017). The average age, body mass index and other parameters during COS of the antagonist and agonist groups were similar without significant difference. Similarly, no significant difference among the three different menstrual phase groups regarding the demographic characteristics is listed in Supplementary Table SII.
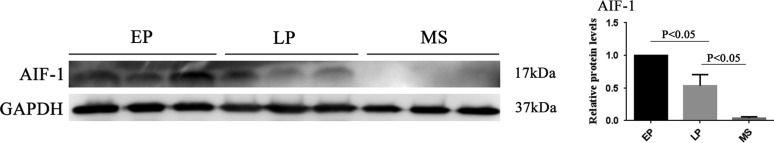
AIF-1 expression within menstrual cycle
AIF-1 expression was high in the early proliferative phase but decreased in the late proliferative phase and almost disappeared in the mid-secretory phase (Fig. 1).
Figure 1.
Representative images and quantification of AIF-1 in endometrial tissue from fertile women at different phases in the menstrual cycle (n = 6 per group) using Western blotting. All data are presented as mean values ± SEM. EP, early proliferative phase; LP, late proliferative phase; MS, mid-secretory phase. The differences between groups were analyzed by Kruskal–Wallis test, followed by multiple comparisons using Mann–Whitney U-test. Data were considered statistically significant if P < 0.05.
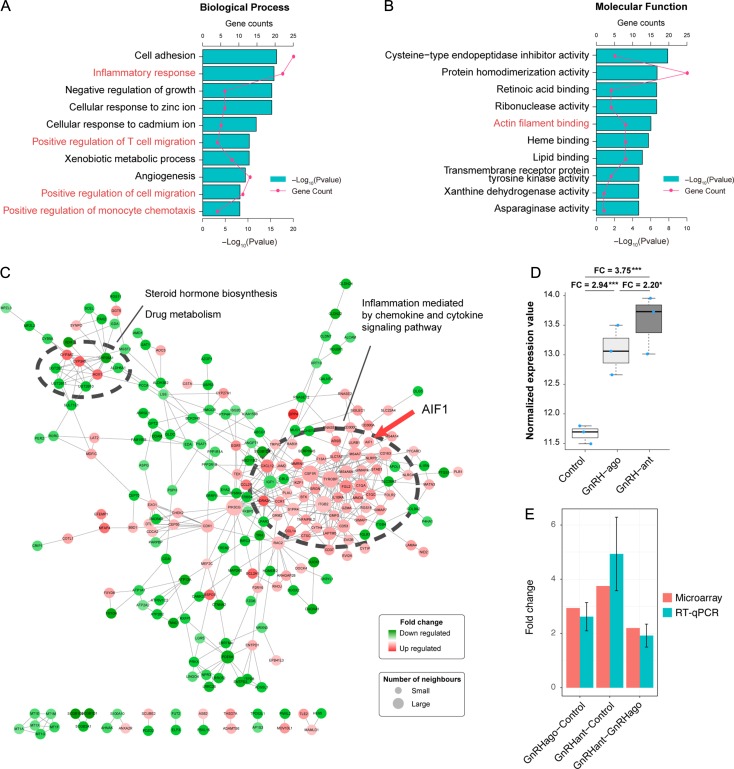
AIF-1 microarray expression and validation by RT-qPCR
The results of function enrichment analysis of differentially expressed genes identified by the microarray analysis showed that AIF-1 was included in GO terms referring to inflammatory response, monocyte chemotaxis and regulating cell migration (Fig. 2A and B). AIF-1 was contained in the module of immune activation (Fig. 2C). The FC of AIF-1 was 3.75 in the antagonist group compared with natural group (P < 0.01) and was 2.20 (P = 0.03) when compared with the agonist group (Fig. 2D). Both results were verified by RT-qPCR on nine samples (Fig. 2E). We have uploaded the raw microarray data and complete gene expression table to GEO under the accession number of GSE107914 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107914) and full gene list was included in Supplementary Table SIII.
Figure 2.
AIF-1 microarray expression and validation (n = 3 per group). (A and B) Functional enrichment results of differentially expressed genes (DEGs), including Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and gene ontology (GO). P-values and gene count of each term are shown as bar plots and lines, respectively. Red text indicates that AIF-1 was among the genes identified. (C) Protein and protein interaction network. Red nodes indicate upregulated proteins, and green nodes indicate downregulated proteins. The size of the node was positively correlated with the quantity of related genes and the larger module was associated with immune activation and contained AIF-1 (red arrow). (D) AIF-1 mRNA expression between the control, GnRH agonist (GnRH ago) and GnRH antagonist (GnRH ant) groups using microarray. ‘FC’ refers to fold change. The y-axis is log 2 scaled and the upper whisker, upper side of box, thick line in the box, lower side of box and the lower whisker corresponds to maximum value, upper 75% quantile, median, lower 25% quantile and minimum value, respectively. (E) AIF-1 mRNA expression in the GnRH agonist/control, GnRH ant/control, and GnRH ant/GnRH agonist using microarray and RT-qPCR analyses. Error bars are expressed as SEM.
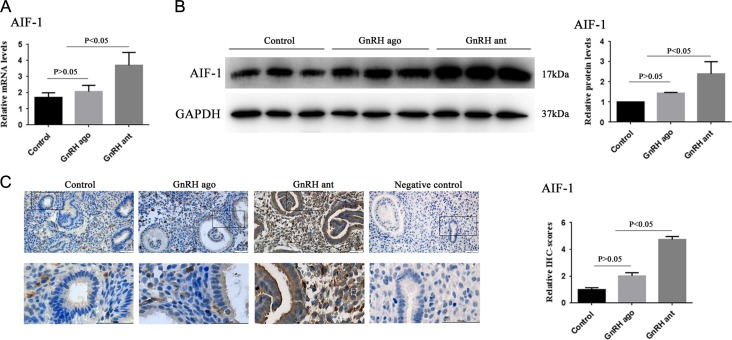
The mRNA and protein expression levels of AIF-1 in three groups
The mRNA and protein expression levels of AIF-1 were significantly increased in the GnRH-ant group compared to the other two groups (P < 0.05), while there were no significant differences between the agonist group and control group (P > 0.05, Fig. 3A and B). The results of the immunohistochemical analysis revealed that AIF-1 was expressed in the endometrial glands and stroma (Fig. 3C), which were consistent with the results from the Western blot analysis (Fig. 3B).
Figure 3.
AIF-1 expression levels in the control group, GnRH agonist group and GnRH antagonist group. (A) Graphs illustrate the relative mRNA level of AIF-1 among the three groups as assayed by RT-qPCR. Analyzed by ANOVA, followed by Bonferroni test. (B) Representative Western blotting images and quantification of AIF-1 protein in the three groups. (C) Representative images and quantification of AIF-1 in the three groups as assessed by immunohistochemistry. All data are presented as mean ± SEM. The differences between groups were analyzed by Kruskal–Wallis test, followed by multiple comparisons using Mann–Whitney U-test in panel B and C. Data were considered statistically significant if P < 0.05.
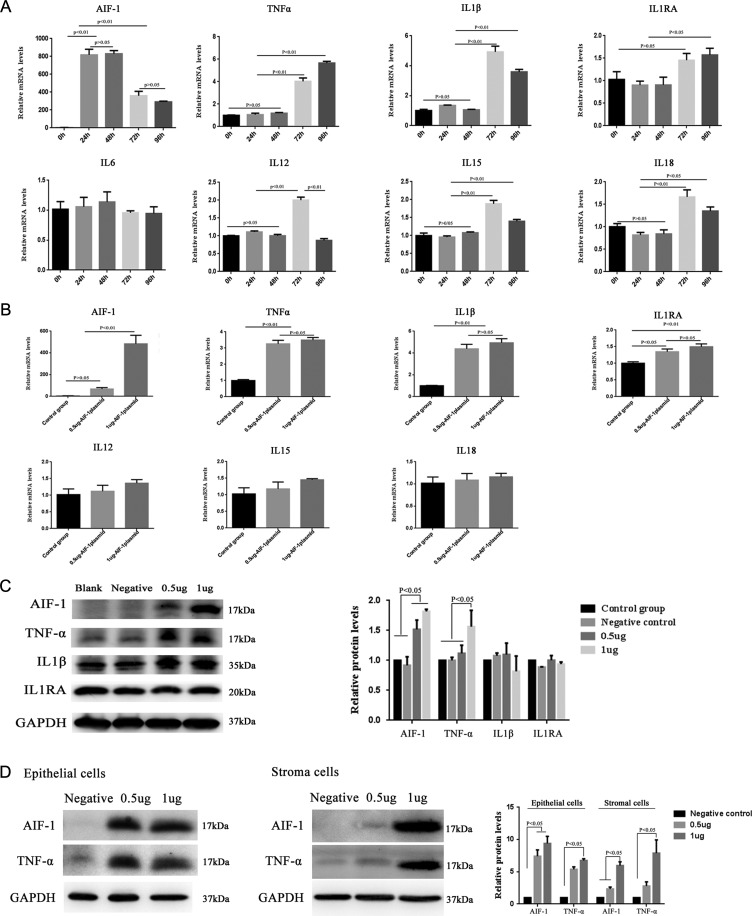
Changes in the expression of cytokines following forced expression of AIF-1 in Ishikawa cells and primary endometrium cells
We measured the expression levels of the related cytokines, such as TNF-α, IL1β, IL1RA, IL12, IL15, IL6 and IL18, in Ishikawa cells following transfection with 1 μg of expression vector encoding AIF-1 for 0, 24, 48, 72 or 96 h. As shown in Fig. 4A, the mRNA expression levels of TNF-α, IL1β, IL1RA, IL12, IL15 and IL18 were significantly increased at 72 h, whereas there was no obvious difference in the expression of IL6 (P > 0.05). In cells transfected for 72 h with varying amounts of AIF-1 expression vector (0.5 or 1 μg), the mRNA expression levels of TNF-α, IL1β and IL1RA were significantly increased, whereas there was no marked change in the mRNA expression levels of IL12, IL15 and IL18 (Fig. 4B). The protein expression levels of TNF-α were significantly increased after transfection with the AIF-1 plasmid (0.5 or 1 μg) for 96 h (P < 0.05). However, the protein expression levels of IL1β and IL1RA did not obviously change (P > 0.05, Fig. 4C). Furthermore, the expression levels of TNF-α were significantly upregulated after transfection of AIF-1 expression vector for 96 h in both primary endometrium epithelial and stromal cells (Fig. 4D). The validation of primary endometrial cells is presented in Supplementary Fig. S1.
Figure 4.
Changes in cytokine levels following force-expression of AIF-1. (A) TNF-α, IL1β, IL1RA, IL6, IL12, IL15 and IL18 mRNA levels assayed by RT-qPCR at 0, 24, 48, 72 and 96 h after transfection with AIF-1-expression vector. All the cytokines increased significantly (P < 0.05 or P < 0.01), except for IL6. (B) Levels of TNF-α, IL1β, IL1RA, IL12, IL15 and IL18 mRNA after 72 h of transfection with AIF-1-expression vector (0.5 or 1 μg). TNF-α, IL1β and IL1RA mRNA levels were significantly higher than the control (P < 0.01). (C) Changes in TNF-α, IL1β and IL1RA protein levels analyzed by Western blotting at 96 h after transfection with the AIF-1-expression plasmid in Ishikawa cells. Only TNF-α was significantly increased (P < 0.05). (D) Changes of TNF-α protein level analyzed by Western blotting at 96 h after transfection with the AIF-1-expression plasmid in primary endothelial and stromal cells. TNF-α was significantly increased after AIF-1 plasmid transfection (P < 0.05). All data are presented as the mean ± SEM. The differences between groups of all data were analyzed by ANOVA, followed by multiple comparisons using Bonferroni test (except AIF-1 expression in Figure 4B, which was analyzed by Kruskal–Wallis test, followed by multiple comparisons using Mann–Whitney U-test). Data were considered statistically significant if P < 0.05.
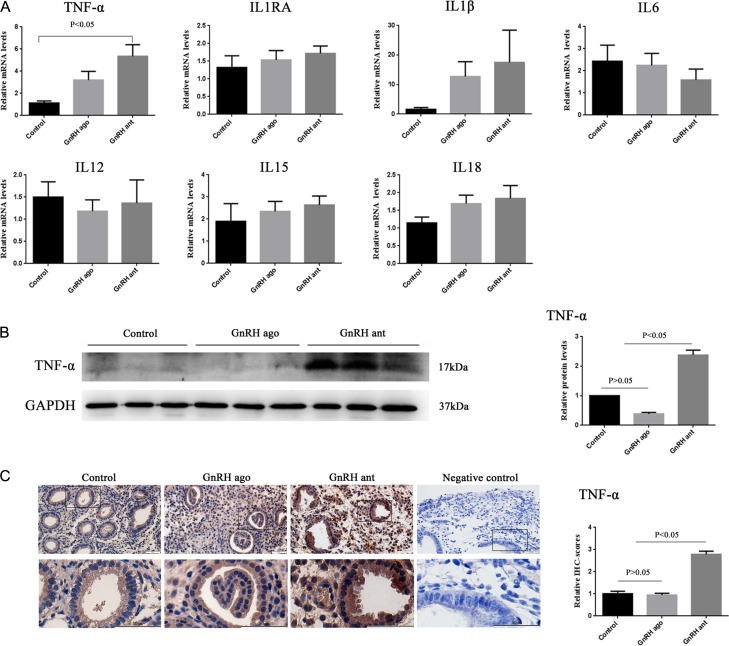
Changes in the expression of related cytokines among the three groups
The mRNA expression levels of the IL1RA, IL1 β, IL12, IL15, IL18 and IL6 did not significantly change among the three groups (Fig. 5A). The mRNA and protein expression levels of TNF-α were significantly increased in the GnRH-ant group compared to those in the other two groups, while there were no significantly different between the agonist group and control group (Fig. 5A–C).
Figure 5.
The expression of cytokines in the control, GnRH agonist, and GnRH antagonist groups. (A) Graphs illustrate the relative mRNA levels of TNF-α, IL1RA, IL1 β, IL6, IL12, IL15, IL18 among the three groups as assayed by RT-qPCR. (B) Representative western blotting images and quantification of TNF-α protein in the three groups. (C) Representative images and quantification of TNF-α in the three groups as assessed by immunohistochemistry. All data are presented as mean ± SEM. The differences between groups of TNF-α and IL18 were analyzed by Kruskal–Wallis test, followed by multiple comparisons using Mann–Whitney U-test and the differences between groups of IL1RA, IL1 β, IL6, IL12, IL15 were analyzed by ANOVA. Data were considered statistically significant at P < 0.05.
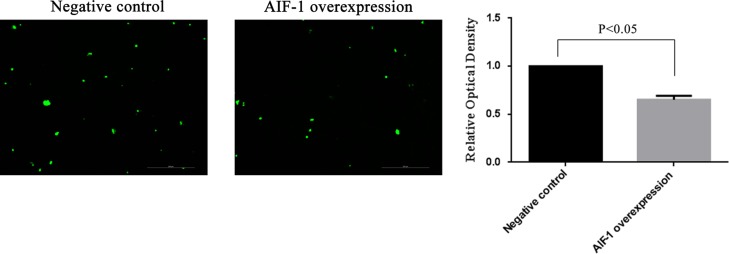
Overexpression of AIF-1 decreased the adhesion of JAR cells onto Ishikawa cells
The fluorescence intensity of fluorescence-labeled JAR cells adherent on AIF-1 overexpressed Ishikawa cells was decreased (up to 0.65-fold) when compared with that on negative lentiviral transduced Ishikawa cells (P < 0.05, Fig. 6). The increased AIF-1 expression in Ishikawa cells after AIF-1 lentiviral transduction was examined using RT-qPCR and western blotting (Supplementary Fig. S2).
Figure 6.
Effect of AIF-1 on the adhesion of JAR cells to Ishikawa cells. Ishikawa cells were treated with negative or AIF-1 expression lentivirus. Fluorescence-labeled JAR cells were calculated and expressed as a fold of negative group. All data are presented as mean ± SEM. The differences between groups were analyzed by independent sample T-test. Data were considered statistically significant if P < 0.05.
Discussion
Many studies including our data show that the GnRH-ant protocol is associated with lower rates of implantation, clinical pregnancy and live birth than the agonist long protocol (Al-Inany et al., 2007; Orvieto et al., 2008; Kdous et al., 2009; Orvieto and Patrizio, 2013; Santana et al., 2014; Kolanska et al., 2017). The latest systematic review showed that, in normal responder patients, no differences in live birth rate were observed although the ongoing pregnancy rate was significantly lower in the antagonist group (Lambalk et al. 2017). Endometrial receptivity has been indentified as a key factor contributing to this situation. As we know, maternal immune tolerance is essential for embryo implantation. AIF-1, an inflammation-responsive scaffold protein and a main factor in allograft rejection, has been identified as a key regulator in manipulating the immune response (Zhou et al., 2011; Zhao et al., 2013). Furthermore, limited previous studies have shown that the high expression level of AIF-1 was associated with uterine allogeneic, resorption-prone pregnancies (Shimada et al., 2003) and endometriosis (Koshiba et al., 2005), indicating that the increased AIF-1 expression might be unfavorable for embryo implantation. However, the relationship between AIF-1 and pregnancy has not been elucidated. Our results showed that the level of AIF-1 showed a dynamic change during the menstrual cycle and reached minimal levels in the mid-secretory phase, a pattern of fluctuation essentially consistent with the study of Koshiba et al. (2005). The results further suggested that during the window phase, the decreased expression of AIF-1 might be in favor of embryo implantation. In addition, our microarray results showed that the expression of AIF-1 was higher in the GnRH-ant group compared to the natural and agonist groups (FC = 3.75 and 2.20, respectively), the results were verified by RT-PCR and western blotting, indicated that the use of GnRH-ant might increase the expression of AIF-1, which may be one of the important reasons of impaired endometrial receptivity.
Then, how does AIF-1 affect endometrial receptivity? In further experiment, we cocultured Ishikawa cells as the endometrial surrogate and JAR cells as the embryo surrogate to simulate the embryo implantation process in vitro (Li et al., 2017) and found that highly expressed AIF-1 could inhibit the adhesion of JAR cells to Ishikawa cells significantly. Adhesion is well known as an important step in trophoblast invasion. These findings suggest that the overexpression of AIF-1 can inhibit the cell adhesion, which is likely to affect endometrial receptivity and consequently lead to reduced implantation and clinical pregnancy rates. We found that the expression of AIF-1 was upregulated in the endometrium in women subject to the GnRH-ant protocol. Meanwhile, it has been reported that GnRH-ant might change the expression of immunomodulatory cytokines related to implantation, such as TNF-α, IL1β, IL6, IL1RA, IL12 and IL15. It has been shown that TNF-α, IL1β, IL1RA and IL12 are involved in the adhesion process during embryo implantation while IL-6 contributes to the placental development. Furthermore, IL15 and IL18 stimulate the activation of uNK cells and regulate inflammatory reaction during implantation (Karmakar et al., 2004; Dimitriadis et al., 2005; Ledee et al., 2011). In order to investigate the effect of increased AIF-1 in GnRH-ant group on implantation process, we performed in vitro experiments to investigate the relationship between AIF-1 and these cytokines using Ishikawa cells. Among those cytokines, only TNF-α was increased in a dose-dependent manner with increasing AIF-1 expression after 96 h of transfection with AIF-1-expressing DNA, which has been verified by the primary endometrial epithelial and stromal cells. These results suggested that TNF-α expression was positively upregulated by AIF-1, which was in accordance with the findings in human primary lung fibroblasts or breast cancer cell lines (Li et al., 2012; Nagahara et al., 2016).
TNF-α is synthesized by both decidual and trophoblast cells and plays an important role in embryo implantation. However, increased secretion of TNF-α is unfavorable to pregnancy and is associated with recurrent miscarriage (Piosik et al., 2013; Zhang et al., 2016) and implantation failure (Liang et al., 2015). Some studies showed that high level of TNF-α can inhibit the adhesion of endometrial epithelial cells to mesothelial cells (Debrock et al., 2006) and thus promote the dyscohesion (cell–cell dissociation) of the endometrial epithelial cells by impairing the expression of adhesion molecule complex cadherin/beta-catenin at the site of cell–cell contact (Tabibzadeh et al., 1995). Todt et al. found that although TNF-α did not alter adhesion of trophoblast cells to laminin or integrin surface expression, however, it significantly inhibited trophoblast cell motility in vitro, suggesting that TNF-α may play an inhibitory role in trophoblast cell invasion (Todt et al., 1996). Interestingly, our results also showed that, similar to AIF-1, the expression of TNF-α was significantly increased in the GnRH-ant group than those in the other two groups. Based on these results, we believe that the increased TNF-α levels mediated by AIF-1 inhibit cell adhesion and further impair endometrial receptivity in IVF cycles with GnRH antagonist protocol.
According to the results, we think that using anti-TNF-α drug might mitigate the adverse effect of GnRH-ant on endometrial receptivity and improve the implantation environment in GnRH-ant protocols. It is worth mentioning that a high progesterone level will impair endometrial receptivity and induce changes at the genomic level (Labarta et al., 2011). In the present study, the average progesterone level on trigger day was a little higher in GnRH-ant group than that in GnRH agonist group, with no significant difference. Therefore, we think the slightly increased progesterone level was not a main reason of GnRH-ant effect on the endometrial receptivity.
In conclusion, our results demonstrate that in GnRH-ant protocol, the increased expression of AIF-1, which further upregulates TNF-α, might have an adverse effect on endometrial receptivity. However, the aberrant AIF-1 and thus TNF-α expression could be one of many factors that may contribute to limiting implantation success. Therefore, further extensive in vitro mechanistic and in vivo animal follow-up studies are needed to assess the actual functional impact of this pathway.
Authors’ roles
A.Z., B.X. and P.C.K.L. devoted most to study design and critical discussion. B.X. and D.Z. recruited the participants and collected the samples. M.Z., J.W. and F.G. made most contributions on experiments execution and manuscript drafting while C.S. and D.Z. made most contributions to data analysis. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China, grant numbers 81771656 and 81370763; Clinical research special fund of Chinese Medical Association, grant number 16020480664; Shanghai Jiao Tong University Medicine-Engineering Fund, Grant number YG2017ZD11 and YG2017MS57; and the Merck-Serono China Research Fund for Fertility Agreement. P.C.K.L. is supported by a Canadian Institutes of Health Research Foundation Scheme Grant 143317.
Conflict of interest
None of the authors have any competing interests.
Supplementary Material
References
- Al-Inany HG, Abou-Setta AM, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception: a Cochrane review. Reprod Biomed Online 2007;14:640–649. [DOI] [PubMed] [Google Scholar]
- Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev 2016;4:Cd001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota AM, Oliveira JB, Petersen CG, Mauri AL, Massaro FC, Silva LF, Nicoletti A, Cavagna M, Baruffi RL, Franco JG Jr. GnRH agonist versus GnRH antagonist in assisted reproduction cycles: oocyte morphology. Reprod Biol Endocrinol 2012;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrock S, De Strooper B, Vander Perre S, Hill JA, D’Hooghe TM. Tumour necrosis factor-alpha, interleukin-6 and interleukin-8 do not promote adhesion of human endometrial epithelial cells to mesothelial cells in a quantitative in vitro model. Hum Reprod 2006;21:605–609. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update 2005;11:613–630. [DOI] [PubMed] [Google Scholar]
- HJ C, TW C, MJ P, YS J, SO L, KJ K, KT H. Water-extracted tubers of Cyperus rotundus L. enhance endometrial receptivity through leukemia inhibitory factor-mediated expression of integrin αVβ3 and αVβ5. J Ethnopharmacol 2017;208:16–23. [DOI] [PubMed] [Google Scholar]
- Huirne JA, Homburg R, Lambalk CB. Are GnRH antagonists comparable to agonists for use in IVF? Hum Reprod 2007;22:2805–2813. [DOI] [PubMed] [Google Scholar]
- Karmakar S, Dhar R, Das C. Inhibition of cytotrophoblastic (JEG-3) cell invasion by interleukin 12 involves an interferon gamma-mediated pathway. J Biol Chem 2004;279:55297–55307. [DOI] [PubMed] [Google Scholar]
- Kdous M, Chaker A, Bouyahia M, Zhioua F, Zhioua A. Increased risk of early pregnancy loss and lower live birth rate with GNRH antagonist vs. long GNRH agonist protocol in PCOS women undergoing controlled ovarian hyperstimulation. Tunis Med 2009;87:834–842. [PubMed] [Google Scholar]
- Kolanska K, Cohen J, Bendifallah S, Selleret L, Antoine JM, Chabbert-Buffet N, Darai E, d’Argent EM. Pregnancy outcomes after controlled ovarian hyperstimulation in women with endometriosis-associated infertility: GnRH-agonist versus GnRH-antagonist. J Gynecol Obstet Hum Reprod 2017;46:681–686. [DOI] [PubMed] [Google Scholar]
- Koshiba H, Kitawaki J, Teramoto M, Kitaoka Y, Ishihara H, Obayashi H, Ohta M, Hara H, Adachi T, Honjo H. Expression of allograft inflammatory factor-1 in human eutopic endometrium and endometriosis: possible association with progression of endometriosis. J Clin Endocrinol Metab 2005;90:529–537. [DOI] [PubMed] [Google Scholar]
- Labarta E, Martinez-Conejero JA, Alama P, Horcajadas JA, Pellicer A, Simon C, Bosch E. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod 2011;26:1813–1825. [DOI] [PubMed] [Google Scholar]
- Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, van der Veen F, van Wely M. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update 2017;23:560–579. [DOI] [PubMed] [Google Scholar]
- Ledee N, Petitbarat M, Rahmati M, Dubanchet S, Chaouat G, Sandra O, Perrier-d’Hauterive S, Munaut C, Foidart JM. New pre-conception immune biomarkers for clinical practice: interleukin-18, interleukin-15 and TWEAK on the endometrial side, G-CSF on the follicular side. J Reprod Immunol 2011;88:118–123. [DOI] [PubMed] [Google Scholar]
- Li T, Feng Z, Jia S, Wang W, Du Z, Chen N, Chen Z. Daintain/AIF-1 promotes breast cancer cell migration by up-regulated TNF-alpha via activate p38 MAPK signaling pathway. Breast Cancer Res Treat 2012;131:891–898. [DOI] [PubMed] [Google Scholar]
- Li HWR, Li YX, Li TT, Fan H, Ng EH, Yeung WS, Ho PC, Lee KF. Effect of ulipristal acetate and mifepristone at emergency contraception dose on the embryo-endometrial attachment using an in vitro human trophoblastic spheroid and endometrial cell co-culture model. Hum Reprod 2017;32:2414–2422. [DOI] [PubMed] [Google Scholar]
- Liang PY, Yin B, Cai J, Hu XD, Song C, Wu TH, Zhao J, Li GG, Zeng Y. Increased circulating Th1/Th2 ratios but not other lymphocyte subsets during controlled ovarian stimulation are linked to subsequent implantation failure after transfer of in vitro fertilized embryos. Am J Reprod Immunol 2015;73:12–21. [DOI] [PubMed] [Google Scholar]
- Meng Y, Guo Y, Qian Y, Guo X, Gao L, Sha J, Cui Y, Chian RC, Liu J. Effects of GnRH antagonist on endometrial protein profiles in the window of implantation. Proteomics 2014;14:2350–2359. [DOI] [PubMed] [Google Scholar]
- Nagahara H, Yamamoto A, Seno T, Obayashi H, Kida T, Nakabayashi A, Kukida Y, Fujioka K, Fujii W, Murakami K et al. . Allograft inflammatory factor-1 in the pathogenesis of bleomycin-induced acute lung injury. Biosci Trends 2016;10:47–53. [DOI] [PubMed] [Google Scholar]
- Orvieto R, Meltzer S, Rabinson J, Zohav E, Anteby EY, Nahum R. GnRH agonist versus GnRH antagonist in ovarian stimulation: the role of endometrial receptivity. Fertil Steril 2008;90:1294–1296. [DOI] [PubMed] [Google Scholar]
- Orvieto R, Patrizio P. GnRH agonist versus GnRH antagonist in ovarian stimulation: an ongoing debate. Reprod Biomed Online 2013;26:4–8. [DOI] [PubMed] [Google Scholar]
- Piosik ZM, Goegebeur Y, Klitkou L, Steffensen R, Christiansen OB. Plasma TNF-alpha levels are higher in early pregnancy in patients with secondary compared with primary recurrent miscarriage. Am J Reprod Immunol 2013;70:347–358. [DOI] [PubMed] [Google Scholar]
- Raga F, Casan EM, Bonilla-Musoles F. Gonadotropin-releasing hormone (GnRH)-I regulation of interleukin (IL)-1b and IL-1 receptor antagonist expression in cultured human endometrial stromal cells. J Obstet Gynaecol Res 2008;34:464–472. [DOI] [PubMed] [Google Scholar]
- Santana R, Setti AS, Maldonado LG, Valente FM, Iaconelli C, Iaconelli A Jr, Jr EB. The impact of pituitary blockage with GnRH antagonist and gonadotrophin stimulation length on the outcome of ICSI Cycles in women older than 36 years. Int J Fertil Steril 2014;8:135–142. [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Iwabuchi K, Watano K, Shimizu H, Yamada H, Minakami H, Onoe K. Expression of allograft inflammatory factor-1 in mouse uterus and poly(I:C)-induced fetal resorption. Am J Reprod Immunol 2003;50:104–112. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S, Kong QF, Kapur S, Satyaswaroop PG, Aktories K. Tumour necrosis factor-alpha-mediated dyscohesion of epithelial cells is associated with disordered expression of cadherin/beta-catenin and disassembly of actin filaments. Hum Reprod 1995;10:994–1004. [DOI] [PubMed] [Google Scholar]
- Todt JC, Yang Y, Lei J, Lauria MR, Sorokin Y, Cotton DB, Yelian FD. Effects of tumor necrosis factor-alpha on human trophoblast cell adhesion and motility. Am J Reprod Immunol 1996;36:65–71. [DOI] [PubMed] [Google Scholar]
- Xu B, Wang J, Xia L, Zhang D, Wu X, Zhang A. Increased uterine NK cell numbers and perforin expression during the implantation phase in IVF Cycles with GnRH Antagonist Protocol. Sci Rep 2017;7:39912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Deng X, Zhang X, Pan Z, Zhao W, Zhang Y, Li J, Xiao F, Wu H, Tan H et al. . Association between serum TNF-alpha levels and recurrent spontaneous miscarriage: a meta-analysis. Am J Reprod Immunol 2016;75:86–93. [DOI] [PubMed] [Google Scholar]
- Zhang D, Ma C, Sun X, Xia H, Zhang W. S100P expression in response to sex steroids during the implantation window in human endometrium. Reprod Biol Endocrinol 2012;10:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Yan DJ, Chen ZW. Role of AIF-1 in the regulation of inflammatory activation and diverse disease processes. Cell Immunol 2013;284:75–83. [DOI] [PubMed] [Google Scholar]
- Zhou X, He Z, Henegar J, Allen B, Bigler S. Expression of allograft inflammatory factor-1 (AIF-1) in acute cellular rejection of cardiac allografts. Cardiovasc Pathol 2011;20:e177–e184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.