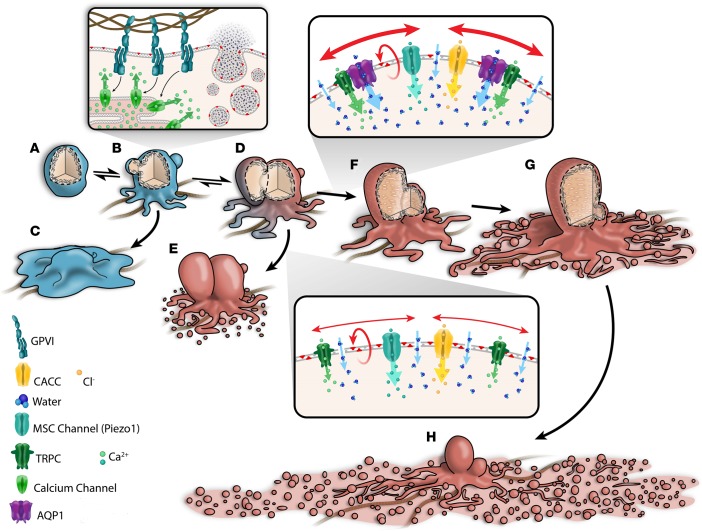
Figure 7. Water entry via aquaporin-1 mediates a stretch-induced amplification of calcium entry and a full procoagulant response in collagen-activated adherent platelets.
Upon contact with subendothelial collagen, platelets (A) signal via glycoprotein receptor VI to cause a rise in cytosolic calcium and the activation of nonspecific cation channels as well as Ca2+-activated chloride channels (CACC), resulting in an initial salt entry, which is then followed by the influx of water along its concentration gradient. Water channel AQP1 facilitates rapid water entry and cell swelling, leading to stretching of the plasma membrane, which then activates the opening of mechanosensitive cation (MSC) channels. Of these, TRPC6 of the family of transient receptor potential cation (TRPC) channels and Piezo1 channels are likely candidates (39) that open to allow additional influx of extracellular calcium, sustaining the rise in cytosolic calcium (B–D), which is critical for activation of the lipid “scramblase,” leading to PS externalization (6) (D and E and D–H). The internal hydrostatic pressure initiates membrane swelling at regions of high calpain activity (6, 56, 57). In combination with external osmotic pressure, this leads to full-scale irreversible membrane ballooning (D–G and D and E). Ballooning is temporally correlated with the formation of the expansive procoagulant surface, which subsequently breaks up as a result of multiple coalescence events to form PS+ve microvesicles (4, 5, 40). (F–H) Both ballooned nonspread (BNS; F) and ballooned procoagulant-spread platelets (BAPS; H) increase the procoagulant area mediating the acceleration of coagulation at wound sites (4, 5, 40). Data from this study indicate that AQP1 has a specific role in the membrane-swelling events that control procoagulant spreading but not ballooning. Upon ablation of AQP1, platelet swelling kinetics are slowed and unable to or only weakly activate MSC channels for enhanced Ca2+ entry. This results in limited Ca2+ influx and procoagulant spreading, thus favoring the formation of the BNS platelet phenotype (D and E) and limited thrombosis.