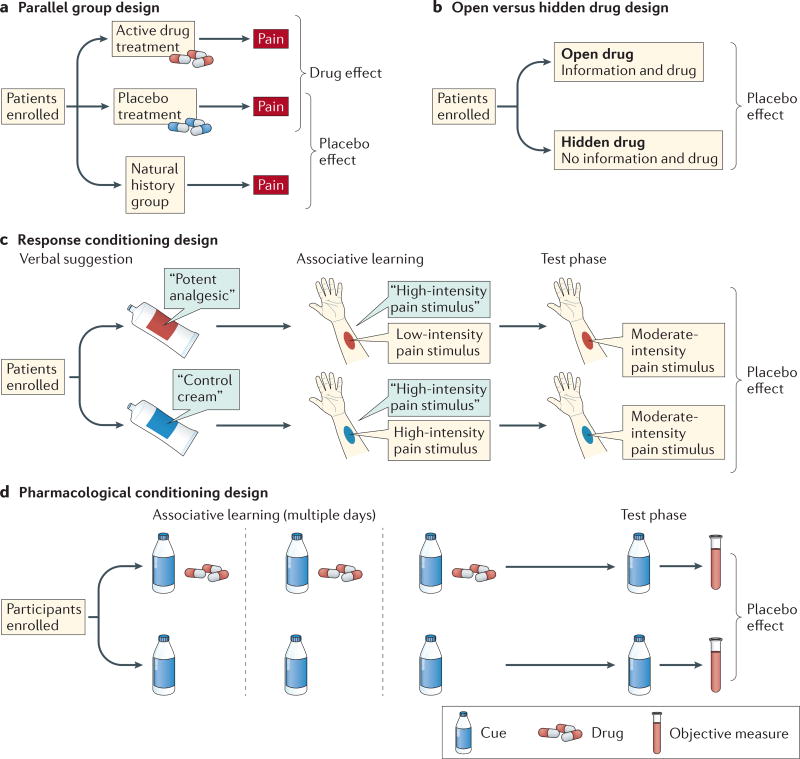
Figure 2. Paradigms for assessing placebo effects.
Most paradigms used to assess placebo treatments fall into one of four categories. a | In a parallel group design, placebo effects are measured by comparing outcomes in a placebo group with those in a no-treatment control group. This is the most common paradigm in clinical trials. b | In an open versus hidden design, drugs are delivered either with (open) or without (hidden) the knowledge of the patient. This design permits assessment of the effects of treatment context in clinical settings without withholding treatment. Extended designs such as the balanced placebo design9 cross open versus hidden administration with verum versus sham drugs, enabling researchers to assess placebo–drug interactions. c | Response conditioning designs use instructions combined with reinforcement to maximize the effectiveness of placebo treatments. In a common variant, initial verbal instructions are provided that one cream (the placebo) is an effective analgesic and another (the control) is not. Then, painful stimulation is given on both placebo-treated and control-treated skin sites. Participants are told that the stimulus intensity will be the same on both sites, but in fact it is surreptitiously reduced for the placebo-treated site, reinforcing belief in the placebo and associations with relief. During a final test phase, equivalent levels of painful stimulation are applied to both sites, and the effects of the placebo conditioning procedure are assessed. This is the most common paradigm used in neuroimaging studies; placebo and control treatments are often compared in a within-person crossover design. d | Pharmacological conditioning designs combine instructions and cues paired with active drugs during a conditioning phase, which often occurs over multiple days. Placebo effects are determined by presenting cues alone and comparing outcomes in drug-paired versus non-drug-paired groups. Response conditioning and pharmacological conditioning designs have been used in both humans and non-human animals.