Abstract
Objective
Electroconvulsive therapy (ECT) is the most robust acute treatment for severe major depressive disorder, yet clinical response is variable. Inflammation is associated with depression, especially in women, and levels of C-reactive protein (CRP) and interleukin-6 (IL-6) predict response to antidepressant medications. This study evaluated whether markers of inflammation predicted response to electroconvulsive therapy (ECT) in patients with treatment resistant depression, and to what extent this association differed between men and women.
Methods
In patients (n=29) with a current major depressive episode diagnosed using DSM-IV-TR criteria, undergoing ECT at an academic referral center, levels of CRP, IL-6, IL-8, tumor necrosis factor (TNF)-α, and severity of depressive symptoms (Montgomery-Asberg Depression Rating Scale, MADRS) were prospectively evaluated before ECT treatment, after the second ECT session, and again at the completion of the index treatment series. Data were collected between 12/2011 and 12/2014. The primary outcome was end-of-treatment MADRS score.
Results
In multivariate analyses, higher levels of IL-6 at baseline, but not other inflammatory markers or clinical variables, were associated with lower end-of-treatment MADRS score (p=0.01). When stratified by sex, IL-6 remained a significant predictor of end-of-treatment MADRS for women (p=0.02) but not men (p=0.1), and CRP emerged as a significant predictor for women (p=0.04) but not men (p=0.66). CRP and IL-6 increased from baseline to the second ECT session (p’s<0.01) and returned to baseline levels at end of treatment; these changes did not relate to MADRS score over the course of ECT.
Conclusions
Levels of IL-6 prior to ECT treatment may be useful in identifying those depressed patients most likely to benefit from ECT treatment. In contrast, acute changes in IL-6 and CRP may reflect spikes in inflammatory response related to the initiation of seizure therapy, but not mood. Assessment of pre-treatment inflammatory biomarkers, especially in women, might be useful in guiding treatment decision-making in treatment resistant depression.
Keywords: Electroconvulsive therapy, major depressive disorder, inflammation, biomarker
INTRODUCTION
Substantial evidence has shown that electroconvulsive therapy (ECT) is a highly effective treatment for major depressive disorder.1 Given considerable costs and potential side effects associated with ECT, and the fact that nearly one third of patients fail to respond,2 there is a need to identify those depressed patients who are most likely to benefit from ECT. Unfortunately, clinical variables have been found to be of limited value in predicting response to ECT. Indeed, a meta-analysis evaluating clinical predictors of ECT response found that only longer duration of current depressive episode and medication failure during the current episode were associated with a lower likelihood of response to ECT. 2 There were weak and non-significant associations suggesting that older age and psychotic features might identify those more likely to respond.2 Baseline symptom severity—traditionally conceptualized as a predictor of better response to ECT— might associate with treatment response, but this meta-analytic result was inconclusive due to heterogeneity across studies.2 No prior studies have examined whether inflammatory markers at baseline might be useful in predicting clinical improvement in response to ECT.
Inflammation is associated with depression,3,4 especially in women,5,6 and is implicated as a causal factor in some types of depression, including interferon-α induced depression, and depressive symptoms elicited by experimental administration of endotoxin.5–12 Patients with an “inflammatory subtype” of depression may also differ in response to treatment. Studies demonstrate that depressed patients with elevated inflammation may respond less robustly to antidepressant medications.13–17
No study has evaluated whether levels of inflammation at baseline might predict clinical improvement in response to ECT. However, ECT has been found to alter cytokine levels.18–24 After a single session of ECT, elevated concentrations of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α have been observed, without persistent elevation after repeated treatments20,21. Over a course of ECT, one small study demonstrated that elevated pre-treatment TNF-α levels in depressed patients normalized, so that post-treatment levels of TNF-α were comparable to control subjects24. A recent study found that clinical remission was associated with a decrease in interleukin (IL)-6 over a course of treatment.25 Thus, though there are a few studies evaluating the trajectory of inflammatory change over the course of ECT, the assessment of inflammatory markers as predictors of ECT treatment outcome is conspicuously absent. Moreover, there are no data to our knowledge that have examined sex differences in the association between inflammation and response to treatment. Women are more affectively sensitive to the effects of inflammatory challenge than men,6 and a recent study demonstrated an association of CRP concentrations with depression severity and specific depressive symptoms in women only.26
This study hypothesized that levels of circulating inflammatory markers would be associated with severity of depressive symptoms following ECT. In patients with major depressive disorder, levels of inflammatory markers and severity of depressive symptoms were evaluated before ECT treatment, after the second ECT session, and again at the completion of the index treatment series. We focused on pro-inflammatory cytokines interleukin-6 (IL-6) and TNF-α, and the acute phase protein C-reactive protein (CRP), because these makers are consistently elevated in association with major depression.3,4 We also included assessment of IL-8 and IL-1β, because both of these cytokines have been found in some studies to be associated with depression.27 The primary purpose of this study was to investigate whether baseline levels of inflammation, or changes in markers of inflammation during treatment, might predict severity of depressive symptoms after ECT treatment in depressed patients, taking into account demographic and clinical characteristics previously associated with ECT response.2 Further, we explored whether there were sex differences in these relationships.
SUBJECTS AND METHODS
Participants
Subjects were depressed patients (N=29, 14 males, 15 females) who were scheduled to undergo ECT treatment at the University of California, Los Angeles (UCLA) Resnick Neuropsychiatric Hospital; this study reports on a subsample of those reported on previously for MRI analysis.28 All procedures were approved by the UCLA Institutional Review Board. Written informed consent was obtained from all participants. Data were collected between December 2011 and December 2014.
Inclusion criteria were current major depressive episode, at least two prior major depressive episodes, and failure to respond to at least two prior antidepressant medications. DSM-IV-TR diagnosis of major depressive episode was confirmed by a board certified psychiatrist and by using the Mini-International Neuropsychiatric Interview (M.I.N.I.). 29 This interview provided information about clinical characteristics including duration of current episode. Exclusion criteria were history of alcohol or substance abuse within the past 6 months and/or dependence within the past 12 months, primary psychotic disorder, dementia, serious medical illness, onset of first episode of depression after age 50, prior ECT, and/or other neuromodulation treatment such as vagal nerve stimulation or repetitive transcranial magnetic stimulation within 6 months of the current ECT index treatment series. Prior to receiving ECT, patients were tapered off psychotropic medications including antidepressants and benzodiazepines (48 – 72 hours).
Procedures
Participants completed an index series of ECT treatments with formal clinical assessments and blood sampling at three time points. The three time points were: prior to, but within 24 hours of the first ECT treatment (T1); at follow-up after the 2nd ECT but preceding the 3rd ECT treatment (T2); and within a week of completing the ECT treatment series (T3, approximately 4–6 weeks after 1st treatment). Clinical assessment of depressive symptom severity and blood sampling for pro-inflammatory cytokines and CRP were obtained at each time point.
ECT Treatment
Adhering to the seizure threshold (ST) titration method of ECT treatment administration, after obtaining the ST, ECT treatments were administered at 5x ST for right unilateral (RUL) d’Elia lead placement, using an ultrabrief pulse-width (0.3msec), and at 1.5x ST for bilateral placement, using a brief pulse-width (0.5msec). Out of the 29 patients, twenty received exclusively right unilateral leads, one received both right and left unilateral leads, and 8 received at least one bilateral lead placement. The 8 patients who progressed to bilateral lead placement were less likely to respond (25%) and remit (12.5%). Those with unilateral lead placements responded (76%) and remitted (43%) at higher rates. See Table 1 for overall response and remission rates. For the index series, ECT (5000Q MECTA Corp.) was administered three times a week, for a mean total of 11.5 sessions per subject (range 6–22 sessions), using a standard protocol for anesthesia (methohexital at 1mg/kg dosage) and paralysis (succinylcholine at 1mg/kg dosage).
Table 1.
Baseline Characteristics and Treatment Information
| Variable | Participants (n=29) |
|---|---|
| Demographic Information | |
| Age, mean years (SD) | 42.6 (14.2) |
| Gender (M/F) | 14/15 |
| BMI, mean (SD) | 26.7 (4.5) |
| Education, mean years (SD) | 15.3 (2.6) |
| Clinical Information | |
| Age at depression diagnosis, mean years (SD) | 28.2 (13.1) |
| Current episode duration, mean years (SD) | 2.0 (2.8) |
| Lifetime illness, mean years (SD) | 17.3 (11.4) |
| ECT Treatment Information | |
| Unilateral/bilateral lead placement | 21/8 |
| # of ECT Index sessions, mean (SD) | 11.5 (3.1) |
| # of ECT Index sessions, range | 6 – 22 |
| Unipolar/bipolar depression | 22/7 |
| Responders a, n (%) | 18 (62%) |
| Remitters b, n (%) | 10 (34%) |
Response was defined as a 50% or larger reduction in MADRS score from baseline to end-of-treatment.
Remission was defined as a final MADRS score ≤ 10.
Abbreviations: BMI = body mass index. ECT = electroconvulsive therapy. F = female. M = male. SD = standard deviation.
Clinical assessment of depressive symptom severity
The Montgomery-Asberg Depression Rating Scale (MADRS)30 was collected at each time point. MADRS score at end-of-treatment was used as the continuous outcome measure for assessing relationships with inflammatory biomarkers, as the primary outcome of interest was degree of clinical severity following ECT. Percent change in MADRS score from baseline to end-of-treatment was also assessed as a secondary outcome.
Assessment of inflammation
At three time points before, during, and after ECT, blood samples were obtained. Because of diurnal variations in circulating cytokine levels that confound interpretation, whole blood samples were collected in the morning between 8 a.m. and 11 a.m. in EDTA tubes, chilled on wet ice, and then centrifuged at 4°C. Plasma was harvested into multiple aliquots, and then stored in a −80°C freezer until assay.
Plasma concentrations of pro-inflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α, were measured utilizing a Bio-Plex 200 (Luminex) instrument and a high-sensitivity multiplex immunoassay (Performance High Sensitivity Human Cytokine, R& D Systems, Minneapolis, MN). Data acquisition and analyses were performed with Bio-Plex software v4.1, and a 5-parameter logistic curve fit. As previously described,31 this multiplex assay has excellent intra-assay (<8% coefficient of variation [CV]) and inter-assay (11–16% CV) reproducibility. Multiplex assays were performed on samples diluted 2-fold according to the manufacturer’s protocol. Plasma concentrations of C-reactive protein (CRP) were determined utilizing the Human CRP Quantikine ELISA (R&D Systems) according to the manufacturer’s protocol with the following modifications: samples were diluted 500-fold, and the standard curve was extended to 0.4 ng/mL to obtain a lower limit of detection of 0.2 mg/mL, taking sample dilution into account. Mean intra-assay CV was <3%, inter-assay CV was <7%. All biomarker assays were performed in duplicate, with all samples from a single individual tested on the same assay plate.
Statistical Analyses
Greater than 45% of samples had IL-1β concentrations below the limit of detection of the multiplex assay (0.1–0.4 pg/mL, depending on the specific assay plate), so this cytokine was not included in statistical analyses. All other cytokines were detectable in 100% of samples. For the small proportion (4%) of samples with CRP concentrations below the limit of detection (0.2 mg/L), a value equal to one-half the lower limit (0.1 mg/L) was assigned. For samples with CRP concentrations above the upper limit of the standard curve (>25 mg/L, 5.6%), the estimated extrapolated CRP concentration was utilized. Multiplex assays for pro-inflammatory cytokines were performed in 2 batches with different kit lots (n=15 subjects batch 1, n=14 subjects batch 2). Therefore, cytokine values were first adjusted using regression to remove the modest variability across the two sets of assays (analyses using the unadjusted values generated identical effects). CRP assays were completed in a single batch. As cytokine and CRP data were not normally distributed, we performed a base 10 logarithmic transformation on the data prior to statistical analyses.
Analyses first evaluated whether circulating levels of pro-inflammatory markers changed during treatment, using repeated measures ANOVA. Univariate regression analyses were then used to evaluate baseline biomarker levels and biomarker change (when significant change was identified via repeated measures ANOVA) as predictors of clinical outcome, defined continuously as MADRS score at T3 (end-of-treatment). These analyses were also stratified by sex. Variables with p <0.15 from univariate analyses were further evaluated in a multiple regression model incorporating clinical variables (i.e., duration of current episode, age, symptom severity at baseline) which have been previously suggested to be related to ECT response, to evaluate whether inflammatory markers predicted MADRS scores when adjusting for these clinical variables. One clinical variable previously associated with response to ECT, medication failure during the current episode,2 was an inclusion criterion for this study and thus was not evaluated as a predictor.
We also evaluated mean cytokine differences in responders compared to non-responders and remitters compared to non-remitters, with independent sample t-tests.
All statistical analyses were conducted using the IBM SPSS (Version 23).
RESULTS
Table 1 summarizes patient demographic information, baseline clinical characteristics, and ECT treatment information.
Depressive symptoms and inflammatory markers over the course of ECT
MADRS scores decreased from baseline to the end of treatment (p<0.001). Levels of CRP (p<0.001) and IL-6 (p<0.01) changed over time, both increasing significantly from T1 to T2, and then decreasing significantly by T3, with no significant change from T1 to T3 (paired t-tests: IL-6 T1 to T3, p=0.80; CRP T1 to T3, p= 0.10). See Table 2 for MADRS scores and concentrations of inflammatory markers across treatment.
Table 2.
Depressive Symptoms and Markers of Inflammation Across Time
| Time point 1a Mdn; M (SD) |
Time point 2a Mdn; M (SD) |
Time point 3a Mdn; M (SD) |
ANOVA p-value | |
|---|---|---|---|---|
| MADRS b | 38.0; 40.1 (7.4) | 32; 31.2 (10.2) | 13.0; 17.5 (12.6) | <0.001 |
| IL-6, pg/mL c | 1.0; 1.2 (1.4) | 1.7; 3.4 (7.4) | 1.0; 1.3 (1.8) | <0.01 |
| IL-8, pg/mL c | 3.0; 3.5 (1.9) | 2.9; 3.3 (1.6) | 3.1; 3.3 (1.5) | 0.69 |
| TNF-α, pg/mL c | 6.0; 6.5 (2.8) | 6.0; 6.7 (2.8) | 6.6; 7.8 (7.6) | 0.58 |
| CRP, mg/L c | 0.9; 2.7 (3.7) | 7.7; 15.0 (20.5) | 1.3; 2.9 (4.1) | <0.001 |
Time point 1 = Patient baseline; Time point 2 = After the 2nd ECT; Time point 3 = After completion of the ECT index series.
MADRS scoring: 0–6 no depression; 7–19 mild; 20–34 moderate; >34 severe.
Values were transformed by base-10 logarithm before ANOVA, but original scale medians, means and standard deviation are presented.
Abbreviations: CRP = C-reactive protein. IL = interleukin. MADRS = Montgomery-Åsberg Depression Rating Scale. M = mean. Mdn = Median. SD = standard deviation. TNF-α = tumor necrosis factor-α.
Higher baseline IL-6 predicts lower end-of-treatment (T3) MADRS score
Univariate linear regression analyses showed that higher levels of IL-6 at baseline predicted lower end-of-treatment MADRS scores following ECT (standardized β coefficient = −0.48, p=0.01). Other baseline inflammatory markers, including CRP, were not associated with MADRS score. Of the two inflammatory markers that changed over time, neither change in IL-6 nor CRP was associated with end-of-treatment MADRS scores (Table 3). Results were similar when the secondary outcome, MADRS percent change from baseline to end-of-treatment, was evaluated.
Table 3.
Inflammatory markers and clinical variables as predictors of end-of-treatment MADRS
| Predictor variables | Univariate analysis | Mutlivariate analysis | ||||
|---|---|---|---|---|---|---|
| Standardized regression coefficients | Effect size (sr2) | p-value | Standardized regression coefficients | Effect size (sr2) | p-value | |
| Baseline CRP a (n=29) | −0.21 | 0.04 | 0.28 | - | - | - |
| Women (n=15) | −0.53 | 0.28 | 0.04 | - | - | - |
| Men (n=14) | 0.13 | 0.02 | 0.66 | - | - | - |
| Baseline IL-6 a (n=29) | −0.48 | 0.23 | 0.01 | −0.51 | 0.24 | 0.01 |
| Women (n=15) | −0.60 | 0.35 | 0.02 | - | - | - |
| Men (n=14) | −0.46 | 0.21 | 0.10 | - | - | - |
| Baseline IL-8 a (n=29) | 0.23 | 0.05 | 0.23 | - | - | - |
| Women (n=15) | 0.41 | 0.17 | 0.13 | - | - | - |
| Men (n=14) | 0.03 | <0.01 | 0.91 | - | - | - |
| Baseline TNF-α a (n=29) | −0.13 | 0.02 | 0.51 | - | - | - |
| Women (n=15) | −0.30 | 0.09 | 0.28 | - | - | - |
| Men (n=14) | 0.03 | <0.01 | 0.91 | - | - | - |
| IL-6 change a (n=29) | −0.06 | <0.01 | 0.74 | - | - | - |
| Women (n=15) | −0.09 | 0.01 | 0.76 | - | - | - |
| Men (n=14) | −0.16 | 0.03 | 0.58 | - | - | - |
| CRP change a (n=29) | 0.19 | 0.04 | 0.33 | - | - | - |
| Women (n=15) | 0.40 | 0.16 | 0.15 | - | - | - |
| Men (n=14) | −0.03 | <0.01 | 0.92 | - | - | - |
| Age | - | - | −0.01 | <0.01 | 0.96 | |
| Baseline MADRS score | - | - | −0.06 | <0.01 | 0.73 | |
| Duration of current episode b | - | - | −0.21 | 0.04 | 0.26 | |
The table shows results of univariate and multiple linear regression models evaluating predictors of clinical outcome as indexed by end-of-treatment MADRS score.
Base 10 log transformations were completed prior to analyses.
Truncated at 5 years.
Abbreviations: CRP = C-reactive protein. IL= interleukin. MADRS = Montgomery-Åsberg Depression Rating Scale. TNF-α = tumor necrosis factor-α.
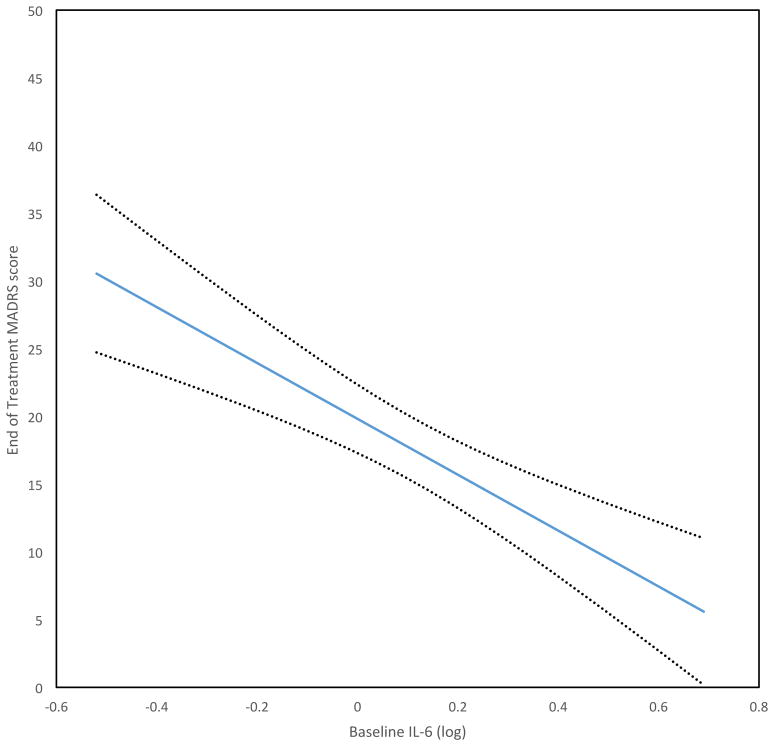
A multiple regression model evaluated whether baseline IL-6 was associated with MADRS scores after ECT, taking into account clinical variables previously suggested to be associated with ECT response (i.e., age, duration of current depressive episode, and symptom severity at baseline). IL-6 remained a significant predictor of end-of-treatment MADRS score (p=0.01), whereas none of the clinical variables were significantly associated with MADRS score (Figure 1; Table 3).
Figure 1.
Higher baseline IL-6 (log) predicts lower end-of-treatment MADRS score, controlling for other variables (age, duration of current episode, baseline depression score; see Table 3). Dotted lines are standard error of prediction.
Mean values of cytokines were evaluated in remitters compared to non-remitters and responders compared to non-responders. Remitters had significantly higher baseline mean values of IL-6 (p=0.046), with a trend in this direction for CRP (p=0.08). Responders also trended toward higher baseline mean values of IL-6 (p=0.14), with no trend observed for CRP.
Relationships between inflammation and final MADRS score were stronger in women
To explore possible sex differences in the relationship between markers of inflammation and MADRS score, further analyses were conducted in which sex was stratified (Table 3). Baseline IL-6 was a significant overall predictor of final MADRS score in women (p=0.02), but only approached a trend in men (p=0.10). Furthermore, whereas baseline CRP was not a significant predictor of end-of treatment MADRS in the total sample (p=0.28), nor for men (p=0.66), CRP was indeed a significant predictor of end-of-treatment MADRS for women (p=0.04). Baseline levels of inflammatory markers did not differ between sexes.
DISCUSSION
This study showed that higher levels of IL-6 prior to treatment with ECT was associated with a more favorable response to treatment, as indexed by lower scores on end-of-treatment MADRS. Importantly, the association between baseline IL-6 and clinical outcome was independent of other clinical variables such as age, baseline severity of depressive symptoms, and duration of current depressive episode. Moreover, this relationship between IL-6 and treatment outcome was stronger in women than in men. Other baseline markers of inflammation were not associated with outcome in the overall sample, but higher baseline CRP was a significant predictor of lower MADRS scores in women, but not men.
IL-6 and CRP both increased from baseline to time point 2, and returned to baseline levels by the end of treatment. These changes were not associated with treatment outcome, and may represent acute stress induced inflammation in response to the initiation of ECT (induction of seizure activity), independent of ECT antidepressant effects. The acute increase of IL-6 following initiation of ECT has been demonstrated in previous studies.20,21 However, two previous studies demonstrated no change in CRP during ECT 23,32, which is surprising given the robust change observed in the current study, and the fact that IL-6 induces transcription of CRP.33
To our knowledge this is the first study to show that baseline IL-6 predicts depressive symptom response to ECT. Our data indicate that depressed patients with higher levels of IL-6 at baseline appear to have lower levels of depressive symptoms in response to ECT. This is similar to findings with ketamine treatment, in which higher levels of IL-6 at baseline were found to predict a more favorable response to ketamine,34 though this was not replicated in a subsequent study.35 In contrast, elevated markers of inflammation are associated with an attenuated response to antidepressant medications.13,14,16,17 Together, these data suggest the possibility that certain treatment interventions for depression may in part be mechanistically dependent on inflammation, or that different subtypes of depression (i.e., inflammatory vs. non-inflammatory) may respond to one treatment approach but not another. Indeed, in studies of deep brain stimulation, an invasive form of neurostimulation, inflammation appears to facilitate early antidepressant effects, as response to treatment was attenuated when non-steroidal anti-inflammatory drugs were given.36
Some have hypothesized that certain basal levels of inflammation are required for antidepressant treatment response,37 and administration of the anti-inflammatory agent infliximab, a TNF-α inhibitor, in treatment resistant depression was only effective in patients with higher levels of CRP (>5 mg/dl); among those with low inflammation, patients showed a worsening of depressive symptoms following administration of infliximab relative to placebo.38
Additional research is necessary to examine the relationships between inflammation and neuroplasticity in the setting of depression. IL-6 concentrations have been positively associated with brain-derived neurotrophic factor (BDNF) concentrations in the setting of MDD with melancholic features,39 and IL-6 has also been shown to enhance secretion of BDNF from monocytes.40 BDNF concentrations may impact neuroplasticity and recovery from depression.41 Given these data, it is possible that elevated levels of IL-6 might identify greater capacity for neuroplasticity through enhanced secretion of BDNF. In the current study, similar to prior studies,20,23 IL-6 increased acutely and transiently in response to ECT. Higher baseline IL-6 and subsequent transient increase in IL-6 concentrations in response to ECT may set the stage for neuroplastic change. Previous findings from our group identified an association between increases in hippocampal volumes and clinical improvement in response to ECT.28 A mechanistic link between inflammation and neuroplasticity might inform understanding of the relationship between this finding and the findings of the current study.
There are several study limitations. Antidepressants and benzodiazepines were discontinued within 48–72 hours of ECT initiation, as per policy at our institution, and it is unknown whether this taper may impact inflammatory markers or ECT treatment outcome. Given the sample size, the study lacks statistical power to examine whether there is a threshold level of IL-6 that might predict clinical response status (i.e., depression remission). Moreover, additional research is necessary to determine whether changes in inflammatory markers might be associated with clinical outcome; a recent study of a similar sample size demonstrated that remitters (in contrast to non-remitters) had a decrease in IL-6 over a course of ECT.25 Finally, biological variability due to sex differences in the association between inflammation and response to ECT needs to be systematically examined to confirm our exploratory findings.
This report provides novel and innovative evidence that higher baseline levels of inflammation as indexed by IL-6 are associated with better clinical outcome following ECT. If this finding is replicated, evaluation of levels of IL-6 might be useful in identifying those depressed patients who might be prioritized for advancement to ECT.
ROLE OF THE FUNDING AGENCY
Research reported in this publication was supported by the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsor was involved in reviewing and approving the study for funding, but not involved in any of the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
CLINICAL POINTS.
Depression is associated with inflammation, and higher inflammation is associated with poorer response to antidepressant medications.
However, we have found that patients with higher blood markers of inflammation (as indexed by IL-6) had greater improvement in depression in response to ECT.
Acknowledgments
Funding/support: This study was supported by grants from the National Institutes of Health to Drs Narr and Espinoza (R01MH092301, K24MH102743, U01MH110008) and to Dr Irwin (R01-AG034588, R01AG026364, R01CA160245-01, R01CA119159, R01HL095799, R01DA032922), and by the Muriel Harris Endowed Chair of Geriatric Psychiatry (Dr Espinoza). The research described was additionally supported by National Institutes of Health/National Center for Advancing Translational Science UCLA CTSI Grant Number UL1TR001881.
The authors thank the UCLA Cousins Center for Psychoneuroimmunology Laboratory for technical support and Christian Perez, BS, of the Inflammatory Biology Laboratory, UCLA Cousins Center for PNI, for the performance of the multiplex assays. Mr Perez has no conflicts of interest to declare.
Footnotes
Previous presentations: Poster presented at the ACNP Annual Meeting, Hollywood, FL, December 4–8, 2016.
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Lisanby SH. Electroconvulsive therapy for depression. The New England journal of medicine. 2007;357(19):1939–1945. doi: 10.1056/NEJMct075234. [DOI] [PubMed] [Google Scholar]
- 2.Haq AU, Sitzmann AF, Goldman ML, Maixner DF, Mickey BJ. Response of depression to electroconvulsive therapy: a meta-analysis of clinical predictors. The Journal of clinical psychiatry. 2015;76(10):1374–1384. doi: 10.4088/JCP.14r09528. [DOI] [PubMed] [Google Scholar]
- 3.Dowlati Y, Herrmann N, Swardfager W, et al. A Meta-Analysis of Cytokines in Major Depression. Biological psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain, behavior, and immunity. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Udina M, Castellvi P, Moreno-Espana J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. The Journal of clinical psychiatry. 2012;73(8):1128–1138. doi: 10.4088/JCP.12r07694. [DOI] [PubMed] [Google Scholar]
- 6.Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40(7):1709–1716. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. The New England journal of medicine. 2001;344(13):961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 8.Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Archives of general psychiatry. 2001;58(5):445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 9.Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain research. 1996;711(1–2):163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 10.Cho HJ, Eisenberger NI, Olmstead R, Breen EC, Irwin MR. Preexisting mild sleep disturbance as a vulnerability factor for inflammation-induced depressed mood: a human experimental study. Translational psychiatry. 2016;6:e750. doi: 10.1038/tp.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological psychiatry. 2010;68(8):748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, behavior, and immunity. 2010;24(4):558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eller T, Vasar V, Shlik J, Maron E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):445–450. doi: 10.1016/j.pnpbp.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine Production and Treatment Response in Major Depressive Disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;22(4):370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 15.Chang HH, Lee IH, Gean PW, et al. Treatment response and cognitive impairment in major depression: association with C-reactive protein. Brain, behavior, and immunity. 2012;26(1):90–95. doi: 10.1016/j.bbi.2011.07.239. [DOI] [PubMed] [Google Scholar]
- 16.Uher R, Tansey KE, Dew T, et al. An Inflammatory Biomarker as a Differential Predictor of Outcome of Depression Treatment With Escitalopram and Nortriptyline. The American journal of psychiatry. 2014 doi: 10.1176/appi.ajp.2014.14010094. [DOI] [PubMed] [Google Scholar]
- 17.Amitai M, Taler M, Carmel M, et al. The Relationship Between Plasma Cytokine Levels and Response to Selective Serotonin Reuptake Inhibitor Treatment in Children and Adolescents with Depression and/or Anxiety Disorders. Journal of child and adolescent psychopharmacology. 2016;26(8):727–732. doi: 10.1089/cap.2015.0147. [DOI] [PubMed] [Google Scholar]
- 18.van Buel EM, Patas K, Peters M, Bosker FJ, Eisel ULM, Klein HC. Immune and neurotrophin stimulation by electroconvulsive therapy: is some inflammation needed after all[quest] Translational psychiatry. 2015;5:e609. doi: 10.1038/tp.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guloksuz S, Rutten BP, Arts B, van Os J, Kenis G. The immune system and electroconvulsive therapy for depression. The journal of ECT. 2014;30(2):132–137. doi: 10.1097/YCT.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 20.Lehtimaki K, Keranen T, Huuhka M, et al. Increase in plasma proinflammatory cytokines after electroconvulsive therapy in patients with depressive disorder. The journal of ECT. 2008;24(1):88–91. doi: 10.1097/YCT.0b013e3181571abb. [DOI] [PubMed] [Google Scholar]
- 21.Fluitman SB, Heijnen CJ, Denys DA, Nolen WA, Balk FJ, Westenberg HG. Electroconvulsive therapy has acute immunological and neuroendocrine effects in patients with major depressive disorder. Journal of affective disorders. 2011;131(1–3):388–392. doi: 10.1016/j.jad.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 22.Zincir S, Ozturk P, Bilgen AE, Izci F, Yukselir C. Levels of serum immunomodulators and alterations with electroconvulsive therapy in treatment-resistant major depression. Neuropsychiatr Dis Treat. 2016;12:1389–1396. doi: 10.2147/NDT.S106652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rush G, O’Donovan A, Nagle L, et al. Alteration of immune markers in a group of melancholic depressed patients and their response to electroconvulsive therapy. Journal of affective disorders. 2016;205:60–68. doi: 10.1016/j.jad.2016.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hestad KA, Tonseth S, Stoen CD, Ueland T, Aukrust P. Raised plasma levels of tumor necrosis factor alpha in patients with depression: normalization during electroconvulsive therapy. The journal of ECT. 2003;19(4):183–188. doi: 10.1097/00124509-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Jarventausta K, Sorri A, Kampman O, et al. Changes in interleukin-6 levels during electroconvulsive therapy may reflect the therapeutic response in major depression. Acta psychiatrica Scandinavica. 2017;135(1):87–92. doi: 10.1111/acps.12665. [DOI] [PubMed] [Google Scholar]
- 26.Köhler-Forsberg O, Buttenschøn HN, Tansey KE, et al. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain, behavior, and immunity. doi: 10.1016/j.bbi.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature reviews Immunology. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi SH, Espinoza RT, Pirnia T, et al. Structural Plasticity of the Hippocampus and Amygdala Induced by Electroconvulsive Therapy in Major Depression. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 30.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. The British journal of psychiatry : the journal of mental science. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 31.Epstein MM, Breen EC, Magpantay L, et al. Temporal stability of serum concentrations of cytokines and soluble receptors measured across two years in low-risk HIV-seronegative men. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(11):2009–2015. doi: 10.1158/1055-9965.EPI-13-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giltay EJ, Kho KH, Blansjaar BA. Serum markers of brain-cell damage and C-reactive protein are unaffected by electroconvulsive therapy. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2008;9(3):231–235. doi: 10.1080/15622970701310989. [DOI] [PubMed] [Google Scholar]
- 33.Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J-j, Wang N, Yang C, Shi J-y, Yu H-y, Hashimoto K. Serum Interleukin-6 Is a Predictive Biomarker for Ketamine’s Antidepressant Effect in Treatment-Resistant Patients With Major Depression. Biological psychiatry. 2015;77(3):e19–e20. doi: 10.1016/j.biopsych.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Park M, Newman LE, Gold PW, et al. Change in cytokine levels is not associated with rapid antidepressant response to ketamine in treatment-resistant depression. Journal of psychiatric research. 2017;84:113–118. doi: 10.1016/j.jpsychires.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez-Caballero L, Perez-Egea R, Romero-Grimaldi C, et al. Early responses to deep brain stimulation in depression are modulated by anti-inflammatory drugs. Molecular psychiatry. 2014;19(5):607–614. doi: 10.1038/mp.2013.63. [DOI] [PubMed] [Google Scholar]
- 37.Yirmiya R, Rimmerman N, Reshef R. Depression as a Microglial Disease. Trends in Neurosciences. 2015;38(10):637–658. doi: 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patas K, Penninx BW, Bus BA, et al. Association between serum brain-derived neurotrophic factor and plasma interleukin-6 in major depressive disorder with melancholic features. Brain, behavior, and immunity. 2014;36:71–79. doi: 10.1016/j.bbi.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Schulte-Herbruggen O, Nassenstein C, Lommatzsch M, Quarcoo D, Renz H, Braun A. Tumor necrosis factor-alpha and interleukin-6 regulate secretion of brain-derived neurotrophic factor in human monocytes. J Neuroimmunol. 2005;160(1–2):204–209. doi: 10.1016/j.jneuroim.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 41.Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484) Molecular psychiatry. 2014;19(7):791–800. doi: 10.1038/mp.2013.105. [DOI] [PubMed] [Google Scholar]