Figure 2.
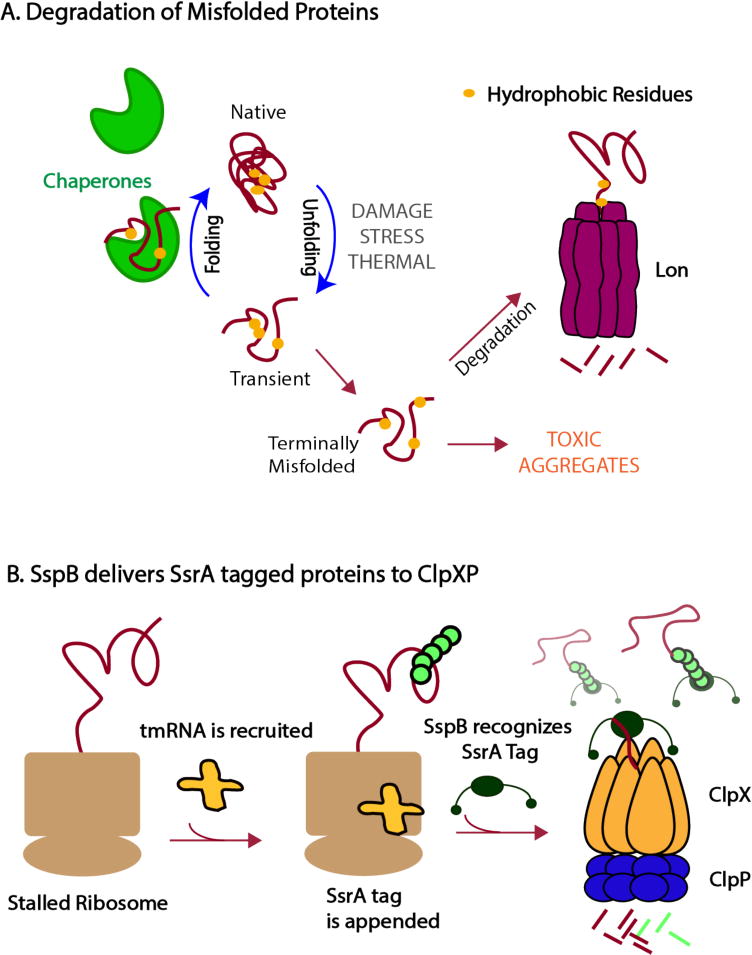
Proteases can survey protein quality in the cell (a) Competition between chaperones and proteases dictate the fate of proteins. Proteases, such as Lon, must be able to distinguish between normal protein dynamics with transient excursions into non-native states and terminally misfolded proteins that must be degraded before forming toxic aggregates. Lon recognizes hydrophobic motifs (shown as yellow circles) that are usually buried in the core of a native protein. These motifs are exposed more persistently for misfolded proteins than during the transient fluctuations of properly folded proteins, allowing Lon to recognize and degrade the terminally misfolded proteins. Chaperones contribute to this flux by binding misfolded proteins in an effort to refold them. (b) Following the process of trans-translation, in which a ssrA-tag is appended to incomplete polypeptides, the adaptor SspB binds tagged substrates and tethers them to ClpXP, enhancing the protease’s ability to degrade these substrates.
