Figure 3.
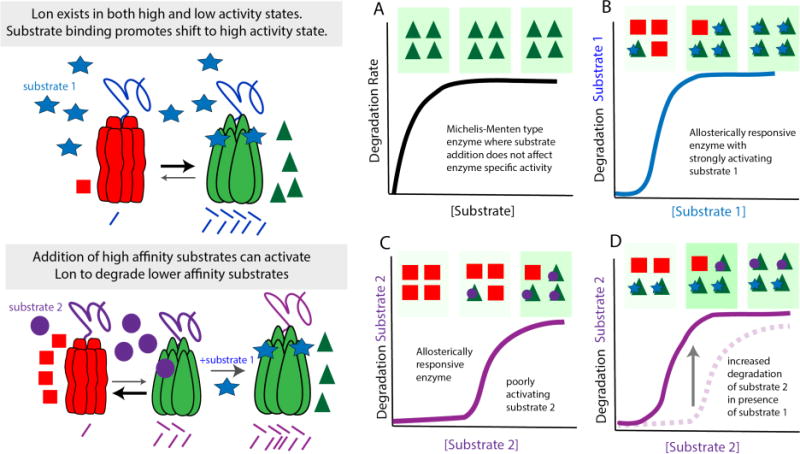
Lon is subject to allosteric regulation (a) Many proteases, such as ClpXP and ClpAP, adhere to typical Michaelis-Menten kinetics where adding increasing amounts of substrate leads to an increase in the rates of degradation until the protease becomes saturated and Vmax is reached resulting in the classic hyperbolic curve. (b) This contrasts with Lon which exhibits positive cooperativity upon increasing substrate concentration. The working model is that Lon exists as low (red triangles) and high activity (green squares) states with substrate binding promoting the highly active state. (b) In the case of substrate 1, which has a strong affinity for Lon, relatively low amounts of substrate are needed to shift Lon to the active state. (c) Substrate 2 binds Lon poorly and activation requires much higher concentrations of substrate 2. (d) If the concentration of substrate 2 is kept the same and the high affinity substrate 1 is added, Lon will be shifted to the active form, leading to robust degradation of the normally poorly degraded substrate 2.
