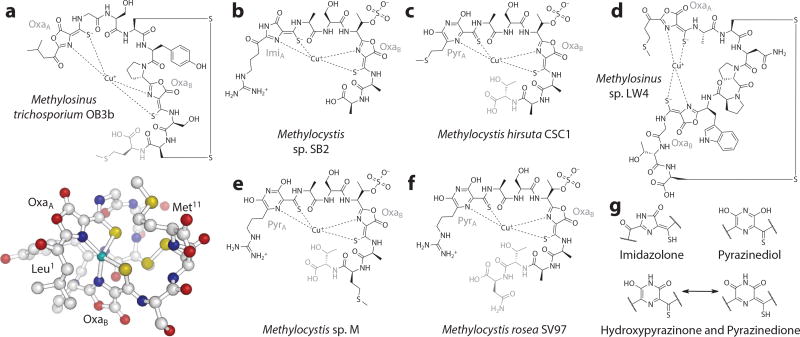
Figure 1.
Structures of methanobactins (Mbns) characterized to date. (a) The structure of copper-chelated Mbn (CuMbn) (top) from Methylosinus (Ms.) trichosporium OB3b. The C-terminal methionine, in gray, is sometimes absent from the final compound. The crystal structure (bottom) clearly shows the distorted tetrahedral geometry of the copper binding site. (b) The structure of CuMbn from Methylocystis (Mc.) sp. SB2. Based on the precursor peptide sequences for this and other Methylocystis Mbns, additional residues are present at the C terminus of these compounds during biosynthesis but have not yet been experimentally observed. (c) The structure of Mbn from Mc. hirsuta CSC1. Determined via X-ray crystallography, this and the remaining Methylocystis Mbns have six-membered rings (assigned as a pyrazinediol) as the first heterocycle. Residues in gray are sometimes absent. (d) The structure of Ms. sp. LW4 CuMbn, which, like Ms. trichosoporium OB3b CuMbn, contains two oxazolone rings and neighboring thioamide groups. (e) The structure of Mbn from Mc. sp. M. Residues in gray are sometimes absent. (f) The structure of CuMbn from Mc. rosea SV97. Residues in gray are sometimes absent. (g) Potential identities for the “N-terminal” heterocycle in Mbns from Methylocystis species. Figure adapted with permission from References 25 and 215.