Abstract
Replacement therapy with missing factor (F) VIII or IX in haemophilia patients for bleed management and preventative treatment or prophylaxis is standard of care. Restoration of thrombin generation through novel mechanisms has become the focus of innovation to overcome limitations imposed by protein replacement therapy. Tissue factor pathway inhibitor (TFPI) is a multivalent Kunitz-type serine protease inhibitor that regulates tissue factor (TF)-induced coagulation through a FXa-dependent feedback inhibition of the TF.FVIIa complex in plasma and on endothelial surfaces. Concizumab is a monoclonal, humanised antibody, specific for the second Kunitz domain of TFPI that binds and inhibits FXa, abolishing the inhibitory effect of TFPI. Concizumab restored thrombin generation in FVIII and FIX deficient plasmas and decreased blood loss in a rabbit haemophilia model. Phase 1 single and multiple dose escalation studies in haemophilia patients demonstrated a dose dependent decrease in TFPI levels and a pro-coagulant effect with increasing d-dimers and prothrombin fragment 1 + 2. A dose dependent increase in peak thrombin and endogenous thrombin potential was observed with values in the normal range when plasma TFPI levels were nearly undetectable. A few haemophilia patients in the highest dose cohorts with complete inhibition of plasma TFPI showed a decreased fibrinogen concentration with normal levels of anti-thrombin and platelets and no evidence of thrombosis. Pharmacokinetic parameters were influenced by binding to the target (TFPI), demonstrating target mediated drug disposition. A trend towards decreasing bleeding tendency was observed and this preventative effect is being studied in Phase 2 studies with additional data gathered to improve our understanding of the therapeutic window and potential for thrombosis.
Key Points for Decision Makers
| Restoration of thrombin generation is increasingly considered as a therapeutic intervention to overcome the limitations of protein replacement therapy. |
| Anti-TFPI monoclonal antibodies restore thrombin generation by abolishing the inhibitory effect of TFPI on the initiation of coagulation. |
| A dose-dependent pro-coagulant effect has been noted in Phase 1 clinical studies with anti-TFPI antibodies with potentially a decrease in bleeding tendency, which requires confirmation in larger studies over a longer duration. |
Introduction
Haemophilia
Haemophilia A and B are inherited bleeding disorders characterised by a deficiency or absence of factor (F) VIII and FIX, respectively. The incidence of haemophilia A is 1 in 5000 male live births, and that of haemophilia B is 1 in 30,000 [1]. The FVIII and FIX Subcommittee of the International Society of Thrombosis and Haemostasis has recommended the use of plasma levels for classifying the severity of haemophilia. Three patient groups are recognised based on their plasma levels: severe haemophilia (FVIII or FIX < 1 IU/dL), moderate haemophilia (FVIII or FIX between 1 and 5 IU/dL) and mild haemophilia (FVIII or FIX between 6 IU/dL and 40 IU/dL) [2]. The classification usually predicts the bleeding phenotype and patients with a severe disorder present with recurrent spontaneous and trauma-related bleeding [2]. In an untreated state, recurrent and spontaneous bleeding into joints and muscles results in disability, with bleeding into vital organs or from a mucosal surface being the most common cause of death [3].
Modern Haemophilia Treatment—Principles and Limitations
Modern haemophilia treatment includes replacement therapy with missing FVIII or FIX with correction of bleeding tendency and a near normal life span [1, 4]. Besides management of bleeds with replacement therapy, regular intravenous infusion either by parents or patients on average between two-to-four times per week improves the bleeding phenotype. This practice of preventive treatment or prophylaxis has been the cornerstone of haemophilia treatment for the last five decades. Prophylaxis, by increasing the baseline level to 1% or greater aims to convert a severe bleeding tendency to a moderate phenotype, thereby decreasing the number of spontaneous bleeds [5].
Different types of prophylactic regimens are recognised based on the timing of prophylaxis initiation. In primary prophylaxis, it is commenced before or after the first joint bleed, but before the second joint bleed. In secondary prophylaxis it is initiated after two or more joint bleeds but before the onset of joint disease, and tertiary prophylaxis is started after the onset of joint disease [6–8]. Primary prophylaxis or early secondary prophylaxis results in near normal joint health and normal life span.
Limitations of current treatment include: implementation of prescribed prophylactic regimens [9], development of inhibitory antibodies that makes treatment ineffective [10], requirement for regular intravenous infusions, difficulties with venous access, patient compliance, cost of drugs, progression of joint disease, risk of intracranial bleeding, and modest treatment goals, all of which impact on quality of life. Further, regular prophylaxis results in oscillation of factor levels and coagulation potential, and while this achieves a minimum trough level of 1% or greater with a decrease in the number of spontaneous bleeds to single figures and potentially to zero, it does not prevent traumatic bleeds. Thus, individual patients require treatment administration to be modified to their personal circumstances, to improve treatment outcomes, potentially increasing treatment burden [11–13].
Current management of bleeding in patients with inhibitors includes treatment with bypassing agents, either recombinant activated factor VIIa (rFVIIa) or activated prothrombin complex concentrate (APCC) [10, 14]. The mechanisms underpinning their haemostatic efficacy are based on restoration of thrombin generation through pathways that potentially contribute a minimal amount of thrombin under normal conditions [15–17]. Besides bleed management, they are used for secondary and tertiary prophylaxis, but they are less effective when compared to FVIII and FIX prophylaxis in non-inhibitor patients and have been associated with occasional episodes of thrombosis [18–21].
Haemophilia Treatment—New Approaches
Restoration of thrombin generation through novel mechanisms has become the focus of innovation in the last decade to overcome some of the limitations imposed by protein replacement therapy. Two broad approaches that restore thrombin generation are in clinical trials. The first approach includes a bi-specific antibody, which acts as a FVIIIa mimetic bridging FIXa and FX with the generation of FXa. The FXa generation is regulated by substrate availability rather than through inhibition of the bi-specific antibody [22, 23]. The second approach results in reduced function of natural inhibitors. This is achieved either through a decrease in activity of anti-thrombin (AT) or tissue factor pathway inhibitor (TFPI). AT is the principle serine protease inhibitor regulating the common, initiation and amplification pathways [24], whereas TFPI is a dominant inhibitor of the initiation pathway [25, 26].
Tissue Factor Pathway Inhibitor—Structure and Distribution
Structure
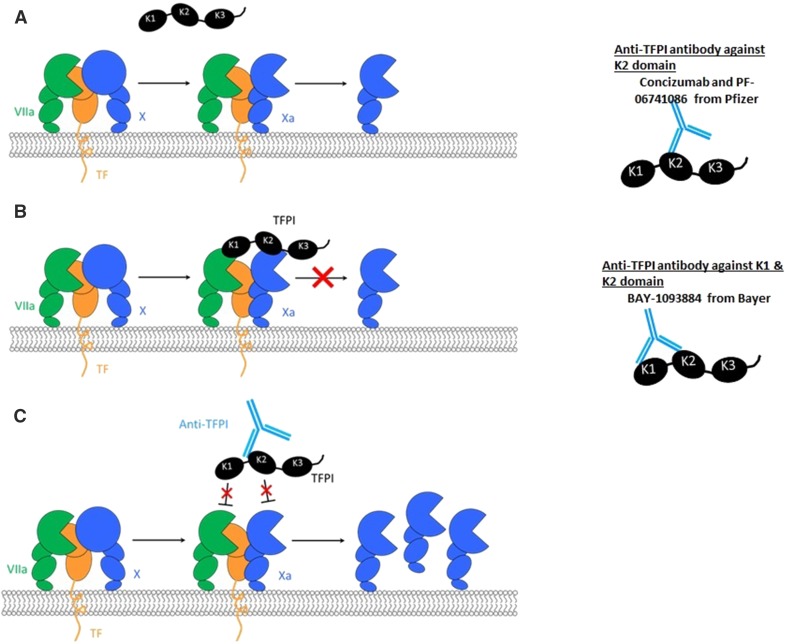
TFPI is a multivalent Kunitz-type serine protease inhibitor that regulates TF-induced coagulation [27] through an FXa-dependent feedback inhibition of the FVIIa. TF complex, which initiates coagulation [27, 28]. TFPI was cloned in 1988 and the amino acid sequence shows a negatively charged amino acid terminus, three tandem Kunitz-type inhibitory domains (K1, K2, and K3), and a positively charged carboxyl terminus [29]. The historical discovery, its role in coagulation regulation and disorders of coagulation have been extensively reviewed [30–32].
Multiple isoforms of TFPI have been described secondary to alternative mRNA splicing events, and the two important isoforms include TFPI alpha (TFPIα) and TFPI beta (TFPIβ) [31]. TFPIα is a 276-residue glycoprotein with an acidic amino terminus followed by three Kunitz domains and a basic carboxy terminus. In TFPIβ, the carboxy terminus contains a glycosylphosphatidylinositol (GPI) anchor replacing the K3 domain [30]. The K1 and K2 domains bind and inhibit FVIIa and FXa, respectively. The K3 domain has no known inhibitory function [30, 31].
Distribution
Endothelial cells and megakaryocytes are the main cells producing TFPI [32, 33]. TFPIβ is the predominant TFPI isoform expressed on endothelium where its association is maintained via a GPI anchor [34, 35]. TFPIα is secreted by human endothelial cells and is present in plasma. TFPI levels are increased two- to four-fold following heparin infusion where TFPIα is the main isoform [30]. This heparin-releasable pool may be bound to cell-surface glycosaminoglycans through its basic C-terminal region, although the exact mechanism is not well described [32]. TFPIα is also produced by megakaryocytes and stored within quiescent platelets. Platelet TFPI is available for release following platelet activation when it can exhibit its inhibitory activity [36]. At the site of vascular injury, local TFPI concentrations appear to increase through the release of TFPI from accumulating platelets within the thrombus and this release is facilitated by dual activation with collagen and thrombin [35].
The mean plasma circulating TFPI concentration in normal individuals is 1.6–2.5 nM or ~ 70 ng/mL [30, 37]. In plasma, 80% of TFPI is predominantly bound to low-density lipoproteins (LDL), and is carboxy-terminal truncated, with levels of the former having an impact on the TFPI levels [30]. The remaining 20% of TFPI circulates in the free form defined by the presence of the K3 domain. It consists of either FL-TFPIα contributing most to the anticoagulant activity or carboxy-terminal truncated TFPI [30]. The platelet TFPI pool exclusively consists of FL-TFPIα, the amount equal to the circulating active full length TFPI. At baseline, the plasma TFPI pool is around 3% of the total vascular TFPI pool. Readily available mature TFPI appears to consist of 95% GPI-anchored TFPIβ [38].
Rationale for Inhibition of TFPI
The blood haemostatic response initiated following damage to the vasculature results in thrombin generation, platelet activation and aggregation, enabling clot formation [39, 40]. This response is localised both spatially and temporally, and localisation is crucial for limiting blood loss without compromising blood flow through excessive clot formation [41, 42]. Inadequate thrombin generation secondary to inherited or acquired deficiency of coagulation factors results in a bleeding diathesis [43]. Replacement therapy or bypass agents restore thrombin generation and clot formation and improve the bleeding tendency [10, 44].
Following tissue injury, the extrinsic pathway or initiation pathway forms the extrinsic tenase complex (TF.FVIIa) and provides the initial FXa for the pro-thrombinase complex. The intrinsic pathway or amplification pathway becomes the source of additional FXa following inhibition of extrinsic tenase complex by TFPI [27, 45]. Pro-thrombinase complex comprimised of FXa bound to activated cellular surfaces in the presence of its co-factor FVa, converts prothrombin to thrombin (common pathway). Thrombin mediates platelet activation and fibrin deposition enabling blood clot formation [41, 42].
The inhibitory activity of TFPI is initiated by the binding of the K2 domain to FXa resulting in its inhibition and the formation of a binary TFPI.FXa complex. This is closely followed by binding of the K1 domain to FVIIa in the TF.FVIIa binary complex to form a quaternary complex that inhibits further activation of FX. The rate-limiting step in these sequential reactions is the inhibition of FXa and not the inhibition of TF.FVIIa [28, 45]. Protein S binds the K3 domain of TFPIα, localising it to membrane surfaces and enhancing the inhibition of FXa by the K2 domain. This is particularly relevant at physiological concentrations of TFPI [46–48]. Further, TFPIα is also able to inhibit FXa-activated FVa and platelet FVa formed in the early stages of clot initiation resulting in inhibition of the pro-thrombinase complex at physiological levels. This interaction is mediated through a high-affinity exosite interaction between the basic region of TFPIα and the FV acidic region [49].
In summary, TFPI inhibits the activity of two major protease–co-factor complexes: extrinsic tenase complex generating FXa and early pro-thrombinase complex that includes FXa activated FVa [31]. Attenuation of this inhibition results in restoration of thrombin generation. The relative contribution of the two pathways in this restoration is not known.
The potential for using TFPI inhibition to manage haemophilia A and B was conceived as early as 1991. In an in vitro laboratory study of haemophilic plasma, a pro-coagulant effect was demonstrated by shortening of the dilute thromboplastin time following the addition of anti-TFPI antibody [50]. A subsequent study tested the clinical effect of a polyclonal anti-TFPI IgG antibody in rabbits with anti-FVIII antibody-induced haemophilia. The bleeding time shortened significantly from 26 to 11 min but did not normalise (normal mean bleeding time in non-haemophilia rabbits: 5 min). Further, correction of coagulation tests was seen at doses of anti-TFPI antibody that were lower than doses required to correct bleeding tendency [51]. Additionally, in a mouse model of haemophilia, an anti-TFPI polyclonal antibody decreased blood loss in a tail clip assay. Blood loss continued to decrease beyond complete inhibition of plasma and endothelial TFPI pools, suggesting a putative role for inhibition of platelet TFPI released at the site of injury [52].
Anti-TFPI Antibodies in Clinical Trials
Three monoclonal antibodies against TFPI are currently in various phases of clinical trials and are illustrated in Fig. 1. Concizumab (mAb-2021) from Novo Nordisk is a monoclonal, humanised IgG4 antibody specific for the K2 domain of TFPI [26]. The explorer™ studies are a series of clinical trials conducted with concizumab as the investigational medicinal product. The studies include explorer 1, a Phase 1 single-dose escalation study [25]; explorer 3, a Phase 1 multiple dose study [53]; explorer 4, a Phase 2 proof of concept multiple-dose study in inhibitor patients (ClinicalTrials.gov Identifier: NCT03196284); and explorer 5, a Phase 2 proof of concept study in non-inhibitor patients (ClinicalTrials.gov Identifier: NCT03196297). BAY-1093884 from Bayer is a monoclonal antibody against both Kunitz-1 and Kunitz-2, presently recruiting to Phase 1 single- and multiple-dose escalation studies (ClinicalTrials.gov Identifier: NCT02571569) [54, 55]. Similarly, PF-06741086 from Pfizer is a monoclonal antibody against the Kunitz-2 domain recruiting to multiple-dose studies in non-inhibitor patients (ClinicalTrials.gov Identifier: NCT02974855) [56].
Fig. 1.
TFPI mechanism of action and inhibition by anti-TFPI antibodies. a Tissue factor (TF) based initiation of coagulation and generation of FXa by the extrinsic tenase complex (FVIIa.TF.FX). b Inhibition of FXa and FVIIa by TFPI. c Binding of the different Kunitz (K) domains by the various anti-TFPI antibodies
Concizumab (mAb 2021)
Following the initial characterisation of TFPI, site-directed mutagenesis confirmed that the K2 domain was required for efficient binding and inhibition of FXa, and both the K1 and K2 were required for inhibition of FVIIa.TF activity. Alterations of the active-site residues of the K3 domain had no significant effect on either function. The production and characterisation of concizumab were detailed by Hilden et al., and a summary is provided below [26].
Isolation and Characterisation
In brief, human recombinant TFPI was isolated from a baby hamster kidney cell line. Mice were subsequently immunised with full-length TFPI and hybridomas were generated from mice splenic cells with aid of myeloma cells and supernatant screened for anti-TFPI antibodies. The anti-TFPI antibodies were subsequently screened for their reactivity against human TFPI. Antibodies of interest were selected based on their ability to inhibit FXa and form a functional quaternary complex. Subsequently, the antibody was humanised and cloned into a human IgG4 format.
X-ray crystallography of concizumab demonstrated an extensive overlap between the binding epitope of concizumab and predicted FXa contact region of the K2 domain, providing a structural basis for the inhibition. Concizumab also demonstrated high affinity binding to both soluble (K2 domain and FL-TFPI) and cell surface bound (human umbilical vein endothelial cells) TFPI.
Ex Vivo Efficacy
The attenuation and abrogation of TFPI activity by concizumab has been demonstrated by the lack of TFPI inhibition of FXa generation in purified systems and cell-based assays. This effect was also demonstrated in a modified prothrombin assay, in which tissue factor (innovin or thromboplastin) concentration was adjusted to give a clot time of ∼ 200 s with normal plasma. A dose-dependent decrease in clotting was observed following the addition of concizumab. Concizumab restored thrombin generation and this was demonstrated in FVIII immune depleted plasma supplemented with platelets at 150 × 109/L. Thrombin generation was activated with low doses of innovin (0.12 pM TF) and dose-dependent changes were observed with increased peak, increased area under curve, and shorter lag times. In whole blood samples from healthy volunteers rendered haemophilic with sheep anti-FVIII IgG, a dose-dependent decrease in clotting time and maximum thrombus formation to within normal ranges was observed when tested by thromboelastography when spiked with concizumab.
In Vivo Efficacy
The in vivo efficacy of concizumab has been demonstrated in a rabbit cuticle bleeding model as the amino acids in the K2 domain of human TFPI defining the antibody-binding epitope are all conserved in rabbit TFPI. Rabbits were induced with haemophilia with an anti-factor VIII antibody. Administration of concizumab significantly reduced cuticle bleeding in haemophilia rabbits when anti-TFPI antibody was administered 30 min prior to induction of bleeding and blood loss was also reduced when administered within 5 min of onset of bleeding. No effect of concizumab was observed when administered 15 or 30 min after induction of bleeding [57]. A dose-dependent effect was noted in the amount of blood loss and duration of effect. Efficacy was also demonstrable after subcutaneous administration.
Concizumab Clinical Studies
Phase 1 Clinical Study
The first in human Phase 1 study (explorer 1) was conducted in patients with severe haemophilia A and B and healthy volunteers. The study design and outcomes are described in brief below [25].
Study Design
The study was a multicentre, randomised, double-blind, placebo-controlled, single-dose, dose-escalation trial. In each dose cohort, trial participants were randomised 3:1 to receive a single dose of concizumab (n = 3) or placebo (n = 1). Following drug administration, patients were seen regularly, and samples were collected for pharmacokinetic (PK), pharmacodynamics (PD), and safety assessments. Initial dosing was performed in healthy volunteers and when pre-defined criteria were reached, the drug was administered to haemophilia patients, thus restricting the number of haemophilia patients required to conduct of the study. The switching criteria were consistent elevation of d-dimers and/or prothrombin fragment 1 + 2 above the normal reference range for 24 h or more in two or more healthy volunteers, and/or when a maximum intravenous (IV) dose of 250 μg/kg and a maximum subcutaneous (SC) dose of 1000 μg/kg was administered to healthy volunteers. Randomised haemophilia patients were in either the IV cohorts (single doses included: 250 , 1000, 3000 and 9000 μg/kg) or in SC cohorts (doses included 1000 and 3000 μg/kg).
Endpoints
The primary endpoint was safety and secondary endpoints included PK and PD parameters. Table 1 describes the various parameters [25].
Table 1.
Study endpoints
| Safety endpoints | Pharmacokinetics (PK)a | Pharmacodynamics (PD)a |
|---|---|---|
| Adverse events over 43 days Clinical assessments including ECG Local injection-site reactions Lab assessments—routine and for evidence of disseminated intravascular coagulation (PT, aPTT, fibrinogen concentration, platelet count, protein C, S & AT activity) Anti-concizumab antibodies by ELISA technique |
PK endpoints—area under curve (AUC), maximum plasma concentration (Cmax), clearance (CL), half-life (t½), mean absorption time, volume of distribution at steady state, bioavailability, and time to maximum concentration Plasma concizumab concentrations (ELISA assay for both free concizumab & concizumab complexed with TFPI) |
D-dimer and prothrombin fragment 1 + 2 concentrations Free TFPI in plasma by ELISA (i.e. TFPI not bound to concizumab, Asserachrom® TFPI) Residual TFPI functionality—detection of FXa generation with chromogenic assay, S2222; Chromogenix) |
aPPT activated partial thromboplastin time, ECG electrocardiogram, TFTI tissue factor pathway inhibitor, PT prothrombin time
aBlood samples for PK and PD analysis were collected at baseline, at 0 (i.e. completion of injection), 5, 15, and 30 min; 1, 4, 8, 12, 24, 36, and 48 h; and 3, 4, 5, 6, 7, 10, 14, 21, and 43 days post-dosing. Blood samples from 5 and 15 min were omitted when the drug was administered subcutaneously
Results—Safety
Fifty-two subjects (28 healthy male volunteers and 24 haemophilia patients: 21 with haemophilia A and 3 with haemophilia B) were enrolled and randomised to treatment or placebo. During the trial, there were no reports of serious adverse events (SAEs). Five of the other reported AEs were possibly or probably treatment related, including two in the placebo group and three in concizumab group. The latter included a single episode of a short segment of superficial thrombophlebitis in a healthy volunteer in the 1000 μg/kg, SC cohort. The patient presented with skin tenderness 5 days post-drug administration and an ultrasound confirmed a short segment of phlebitis. Injection site reactions were seen in a few patients.
In the haemophilia patients randomised to the higher SC dose cohorts (1000 or 3000 μg/kg), transient elevation of troponin T was noted in 3 patients with no significant ECG abnormalities. Similarly, a couple of haemophilia patients randomised to the higher IV dose cohorts (1000 or 3000 μg/kg) halved their fibrinogen concentration with no concurrent decrease in protein C, protein S, AT, or platelet count.
Results—Pharmacokinetics
Concizumab was detected in plasma up to 43 days after dosing. There were no differences in the PK profiles between healthy volunteers and haemophilia subjects. PK profiles were consistent with target-mediated drug disposition (TMDD) in which binding of concizumab to TFPI influenced drug distribution, elimination, and plasma concentration.
Results—Pharmacodynamics
Two assays were used to assess the impact of concizumab. The first was a quantitative assay to determine the total amount of TFPI by ELISA in the plasma that does not bind to the drug. A dose-dependent decrease in concizumab free-total TFPI concentration was observed. The levels were low for up to 2 weeks in the highest dose cohort. The second assay was a functional assay that measured FXa generation as a function of TFPI. The residual functional TFPI levels decreased with the total TFPI levels. A dose-dependent pro-coagulant response as assessed by d-dimer and prothrombin fragment 1 + 2 levels was seen. The steepest increase was observed in healthy volunteers following IV administration. Importantly, haemophilia patients showed similar d-dimer response compared with healthy volunteers when they received an approximately 36-fold higher dose of concizumab.
Thrombin Generation and Concizumab
Ex vivo spiking of samples from patients with haemophilia A and B with concizumab displayed a dose-dependent increase in peak thrombin and endogenous thrombin potential (ETP) with shortening of the lag time. The influence on peak thrombin was more marked when compared with ETP. A similar effect was seen in samples taken from healthy volunteers dosed with concizumab [58].
Clinical Study—Multiple Dose
The findings in explorer 1 study, were replicated in explorer 3, which was a placebo-controlled, multiple-dose, dose-escalation study where concizumab was administered SC [53]. A dose-dependent decrease in concizumab free-total TFPI and procoagulant effect were noted. Further, a dose-dependent increase in peak thrombin and ETP was seen, and in the higher dose cohort, thrombin generation parameters were within normal range.
Clinical Study—Phase 2 Proof of Concept
Both explorer 1 and explorer 3 demonstrated a decreasing bleeding tendency with higher concizumab doses, but the trials were not designed to demonstrate proof of efficacy [25]. explorer 4 and 5 will explore the ability of concizumab to decrease bleeding tendency in patients with severe haemophilia A and B with and without inhibitors, respectively. Due to the significant impact of TMDD, concizumab will be administered daily, and to decrease treatment burden, it will be administered SC [53]. The starting dose in the trials is 0.15 mg/kg (150 μg/kg) with dose escalation to a maximum of 0.25 mg/kg (250 μg/kg). The bleed frequency will be observed for a period of 24 weeks in the first instance, followed by a 12-month extension phase. Patients will continue to administer FVIII or rFVIIa for management of bleeds. In addition to demonstrating efficacy of concizumab, the trials could potentially shed light on the level of TFPI inhibition required for decreasing the bleeding tendency.
Target-Mediated Drug Disposition (TMDD)
TMDD—Definition and Description
The PK profile of small drug molecules is typically linear, with dose increases resulting in a proportional increase in plasma concentration with binding to receptors having a minimal effect. Non-linear PK is also common, where there is a lack of proportional increase in plasma concentration with increases in drug dose. This non-proportional response impacts on the relationship between drug concentration and desired pharmacological effect and undesired toxicity [59]. The term TMDD has been used to describe non-linear PK, where a significant proportion of the drug is bound with high affinity to the pharmacological target, relative to the dose administered [60].
The PK consequences of TMDD are pronounced when binding to the target contributes to significant elimination of the drug. Further, if the binding is saturable it results in different concentration–time profiles for different individual doses [61]. With saturable target binding, at low drug concentration, administration of increasing doses is associated with an apparent decreasing steady-state volume of distribution until the target is saturated. Following saturation of the target binding, a limiting value is reached with a long terminal elimination half-life. Therefore, when target binding contributes significantly to clearance, clearance can decrease with increasing dose [61, 62]. In this context, it is important to identify the drug- and target-specific parameters that influence exposure–response relationships, and patient-specific characteristics that account for inter-subject PD variability [61].
TMDD and Concizumab
To understand the impact of TMDD on the PK profile of concizumab, several experiments were undertaken on Cynomolgus monkeys [62]. A classical TMDD model was used for analysing PK, which included compartments for both standard drug disposition and target binding. Following subcutaneous administration, bioavailability of concizumab was estimated at 93%, absorption half-life was estimated at 72 h and terminal half-life was dependent on plasma concentration. At low levels of concizumab, non-linear clearance was the most important elimination route, secondary to TFPI binding. Maximum elimination rate (Vmax) was estimated at 11 μg/kg/h. When levels reached over 100 μg/mL, conventional linear clearance dominated and was estimated at 0.14 mL/h/kg [62].
TMDD was confirmed in the Phase 1 studies and, at low concizumab concentrations, binding to TFPI was the dominant influence resulting in faster nonlinear clearance; whereas at higher levels, a slower more linear clearance was noted [25].
Challenges of Pro-Coagulant Therapies
A major challenge for the widespread use of pro-coagulant therapies is the lack of data around the overlap between the therapeutic window, where bleed prevention is achieved, and the toxicity window where there is a potential increased risk of thrombosis. Under baseline conditions of regular prophylaxis, such overlap is unlikely. Further, the interactions between replacement therapy and bypass agents administered for bleed management, and the novel pro-coagulant therapies need to be investigated urgently. In this context, transient hypercoagulability is to be expected, but there are limited data on the magnitude, duration, and clinical impact of this hypercoagulability. Further, there has been a tendency towards a one-size-fits-all approach when evidence from thrombin generation studies shows a wide range of values both in the normal population and patients with haemophilia A and B.
Further, the in vivo mechanisms that underpin clot formation and thrombin generation need to be elucidated. Elevated d-dimers represent a pro-coagulant state, but borderline elevations could also potentially represent exaggerated basal coagulation with no impact on either bleeding or thrombotic tendency [63].
Concizumab clinical trials have shown that the doses selected for the Phase 2 studies inhibit most of the plasma pool and a proportion of the intravascular pool, and one could speculate that this might provide protection against thrombosis as most of the intravascular pool is localised at the endothelial surface. Further, there are no routine tests available for monitoring the restoration of thrombin generation in a meaningful fashion. Additional data on the correlation between measures of thrombin generation and clinical effect are required.
Conclusion
In summary, the advent of pro-coagulant therapies aiming to restore thrombin generation through exploitation of non-dominant coagulation pathways raises exciting possibilities of not only decreased treatment burden but also improved patient outcomes. These possibilities need to be tempered against potential thrombotic risks and unanticipated risks, which need to be addressed in forthcoming trials and post-marketing surveillance studies.
Acknowledgments
I would like to thank the staff and patients of Katharine Dormandy Haemophilia and Thrombosis Centre for their participation in trials of anti-TFPI monoclonal antibodies.
Compliance with Ethical Standards
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
PC reports, grants and personal fees from Pfizer, grants, personal fees and non-financial support from Bayer, personal fees and non-financial support from Baxalta (Shire), grants, personal fees and non-financial support from CSL Behring, grants, personal fees and non-financial support from NovoNordisk, grants, personal fees and non-financial support from Swedish Orphan Biovitrum AB (Sobi), personal fees from Freeline, personal fees from Roche, outside the submitted work.
Footnotes
The original version of this article was revised: Due to Figure 1 caption update.
A correction to this article is available online at https://doi.org/10.1007/s40265-018-0933-3.
Change history
6/11/2018
Mechanisms of action of monoclonal antibodies against tissue factor pathway inhibitor (TFPI).
References
- 1.Mannucci PM, Tuddenham EG. The haemophilias–from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773–1779. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- 2.White GC, 2nd, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J. Definitions in haemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001;85(3):560. doi: 10.1055/s-0037-1615621. [DOI] [PubMed] [Google Scholar]
- 3.Larsson SA. Life expectancy of Swedish haemophiliacs, 1831-1980. Br J Haematol. 1985;59(4):593–602. doi: 10.1111/j.1365-2141.1985.tb07353.x. [DOI] [PubMed] [Google Scholar]
- 4.Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388(10040):187–197. doi: 10.1016/S0140-6736(15)01123-X. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson IM, Berntorp E, Lofqvist T, Pettersson H. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232(1):25–32. doi: 10.1111/j.1365-2796.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 6.Ljung R, Gretenkort Andersson N. The current status of prophylactic replacement therapy in children and adults with haemophilia. Br J Haematol. 2015;169(6):777–786. doi: 10.1111/bjh.13365. [DOI] [PubMed] [Google Scholar]
- 7.Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with haemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110(3):815–825. doi: 10.1182/blood-2006-10-050435. [DOI] [PubMed] [Google Scholar]
- 8.Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A. Definitions in haemophilia: communication from the SSC of the ISTH. J Thromb Haemost JTH. 2014;12(11):1935–1939. doi: 10.1111/jth.12672. [DOI] [PubMed] [Google Scholar]
- 9.Richards M, Williams M, Chalmers E, Liesner R, Collins P, Vidler V, Hanley J. A United Kingdom Haemophilia Centre Doctors’ Organization guideline approved by the British Committee for Standards in Haematology: guideline on the use of prophylactic factor VIII concentrate in children and adults with severe haemophilia A. Br J Haematol. 2010;149(4):498–507. doi: 10.1111/j.1365-2141.2010.08139.x. [DOI] [PubMed] [Google Scholar]
- 10.Collins PW, Chalmers E, Hart DP, et al. Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia: (4th edition). UK Haemophilia Centre Doctors Organization. Br J Haematol. 2013;160(2):153–170. doi: 10.1111/bjh.12091. [DOI] [PubMed] [Google Scholar]
- 11.Broderick CR, Herbert RD, Latimer J, et al. Association between physical activity and risk of bleeding in children with haemophilia. JAMA J Am Med Assoc. 2012;308(14):1452–1459. doi: 10.1001/jama.2012.12727. [DOI] [PubMed] [Google Scholar]
- 12.Collins PW. Personalized prophylaxis. Haemophilia. 2012;18(Suppl 4):131–135. doi: 10.1111/j.1365-2516.2012.02838.x. [DOI] [PubMed] [Google Scholar]
- 13.Valentino LA. Considerations in individualizing prophylaxis in patients with haemophilia A. Haemophilia. 2014;20(5):607–615. doi: 10.1111/hae.12438. [DOI] [PubMed] [Google Scholar]
- 14.Hedner U, Lee CA. First 20 years with recombinant FVIIa (NovoSeven) Haemophilia. 2011;17(1):e172–e182. doi: 10.1111/j.1365-2516.2010.02352.x. [DOI] [PubMed] [Google Scholar]
- 15.Giansily-Blaizot M, Schved JF. Recombinant human factor VIIa (rFVIIa) in haemophilia: mode of action and evidence to date. Ther Adv Hematol. 2017;8(12):345–352. doi: 10.1177/2040620717737701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman M, Monroe DM. 3rd The action of high-dose factor VIIa (FVIIa) in a cell-based model of hemostasis. Disease-a-month DM. 2003;49(1):14–21. doi: 10.1053/mda.2003.29504b. [DOI] [PubMed] [Google Scholar]
- 17.Varadi K, Tangada S, Loeschberger M, Montsch P, Schrenk G, Ewenstein B, Turecek PL. Pro- and anticoagulant factors facilitate thrombin generation and balance the haemostatic response to FEIBA((R)) in prophylactic therapy. Haemophilia. 2016;22(4):615–624. doi: 10.1111/hae.12873. [DOI] [PubMed] [Google Scholar]
- 18.Leissinger C, Gringeri A, Antmen B, et al. Anti-inhibitor coagulant complex prophylaxis in haemophilia with inhibitors. N Engl J Med. 2011;365(18):1684–1692. doi: 10.1056/NEJMoa1104435. [DOI] [PubMed] [Google Scholar]
- 19.Leissinger CA, Becton DL, Ewing NP, Valentino LA. Prophylactic treatment with activated prothrombin complex concentrate (FEIBA) reduces the frequency of bleeding episodes in paediatric patients with haemophilia A and inhibitors. Haemophilia. 2007;13(3):249–255. doi: 10.1111/j.1365-2516.2007.01442.x. [DOI] [PubMed] [Google Scholar]
- 20.Franchini M, Coppola A, Tagliaferri A, Lippi G. FEIBA versus NovoSeven in haemophilia patients with inhibitors. Semin Thromb Hemost. 2013;39(7):772–778. doi: 10.1055/s-0033-1354422. [DOI] [PubMed] [Google Scholar]
- 21.Chai-Adisaksopha C, Nevitt SJ, Simpson ML, Janbain M, Konkle BA. Bypassing agent prophylaxis in people with haemophilia A or B with inhibitors. Cochrane Database Syst Reviews (Online) 2017;9:441. doi: 10.1002/14651858.CD011441.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab Prophylaxis in Haemophilia A with Inhibitors. N Engl J Med. 2017;377(9):809–818. doi: 10.1056/NEJMoa1703068. [DOI] [PubMed] [Google Scholar]
- 23.Kitazawa T, Igawa T, Sampei Z, et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a haemophilia A model. Nat Med. 2012;18:1570. doi: 10.1038/nm.2942. [DOI] [PubMed] [Google Scholar]
- 24.Pasi KJ, Rangarajan S, Georgiev P, et al. Targeting of antithrombin in haemophilia A or B with RNAi therapy. N Engl J Med. 2017;377:819–828. doi: 10.1056/NEJMoa1616569. [DOI] [PubMed] [Google Scholar]
- 25.Chowdary P, Lethagen S, Friedrich U, et al. Safety and pharmacokinetics of anti-TFPI antibody (concizumab) in healthy volunteers and patients with haemophilia: a randomized first human dose trial. J Thromb Haemost. 2015;13(5):743–754. doi: 10.1111/jth.12864. [DOI] [PubMed] [Google Scholar]
- 26.Hilden I, Lauritzen B, Sorensen BB, et al. Hemostatic effect of a monoclonal antibody mAb 2021 blocking the interaction between FXa and TFPI in a rabbit haemophilia model. Blood. 2012;119(24):5871–5878. doi: 10.1182/blood-2012-01-401620. [DOI] [PubMed] [Google Scholar]
- 27.Broze GJ, Jr, Warren LA, Novotny WF, Higuchi DA, Girard JJ, Miletich JP. The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action. Blood. 1988;71(2):335–343. [PubMed] [Google Scholar]
- 28.Baugh RJ, Broze GJ, Jr, Krishnaswamy S. Regulation of extrinsic pathway factor Xa formation by tissue factor pathway inhibitor. J Biol Chem. 1998;273(8):4378–4386. doi: 10.1074/jbc.273.8.4378. [DOI] [PubMed] [Google Scholar]
- 29.Wun TC, Kretzmer KK, Girard TJ, Miletich JP, Broze GJ., Jr Cloning and characterization of a cDNA coding for the lipoprotein-associated coagulation inhibitor shows that it consists of three tandem Kunitz-type inhibitory domains. J Biol Chem. 1988;263(13):6001–6004. [PubMed] [Google Scholar]
- 30.Broze GJ, Jr, Girard TJ. Tissue factor pathway inhibitor: structure-function. Front Boisci (Landmark edition) 2012;17:262–280. doi: 10.2741/3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mast AE. Tissue factor pathway inhibitor: multiple anticoagulant activities for a single protein. Arterioscler Thromb Vasc Biol. 2016;36(1):9–14. doi: 10.1161/ATVBAHA.115.305996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood JP, Ellery PE, Maroney SA, Mast AE. Biology of tissue factor pathway inhibitor. Blood. 2014;123(19):2934–2943. doi: 10.1182/blood-2013-11-512764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bajaj MS, Kuppuswamy MN, Saito H, Spitzer SG, Bajaj SP. Cultured normal human hepatocytes do not synthesize lipoprotein-associated coagulation inhibitor: evidence that endothelium is the principal site of its synthesis. Proc Natl Acad Sci USA. 1990;87(22):8869–8873. doi: 10.1073/pnas.87.22.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maroney SA, Ferrel JP, Pan S, White TA, Simari RD, McVey JH, Mast AE. Temporal expression of alternatively spliced forms of tissue factor pathway inhibitor in mice. JThromb Haemost JTH. 2009;7(7):1106–1113. doi: 10.1111/j.1538-7836.2009.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maroney SA, Mast AE. Expression of tissue factor pathway inhibitor by endothelial cells and platelets. Transf Apheresis Sci. 2008;38(1):9–14. doi: 10.1016/j.transci.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maroney SA, Haberichter SL, Friese P, Collins ML, Ferrel JP, Dale GL, Mast AE. Active tissue factor pathway inhibitor is expressed on the surface of coated platelets. Blood. 2007;109(5):1931–1937. doi: 10.1182/blood-2006-07-037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bajaj MS, Kuppuswamy MN, Manepalli AN, Bajaj SP. Transcriptional expression of tissue factor pathway inhibitor, thrombomodulin and von Willebrand factor in normal human tissues. Thromb Haemost. 1999;82(3):1047–1052. [PubMed] [Google Scholar]
- 38.Girard TJ, Tuley E, Broze GJ., Jr TFPIbeta is the GPI-anchored TFPI isoform on human endothelial cells and placental microsomes. Blood. 2012;119(5):1256–1262. doi: 10.1182/blood-2011-10-388512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359(9):938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 40.Kim K, Hahm E, Li J, et al. Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood. 2013;122(6):1052–1061. doi: 10.1182/blood-2013-03-492504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monroe DM, Hoffman M. What does it take to make the perfect clot? Arterioscler Thromb Vasc Biol. 2006;26(1):41–48. doi: 10.1161/01.ATV.0000193624.28251.83. [DOI] [PubMed] [Google Scholar]
- 42.Butenas S, van’t Veer C, Mann KG. “Normal” thrombin generation. Blood. 1999;94(7):2169–2178. [PubMed] [Google Scholar]
- 43.Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33(1):4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 44.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of haemophilia. Haemophilia. 2013;19(1):e1–e47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 45.Girard TJ, Warren LA, Novotny WF, Likert KM, Brown SG, Miletich JP, Broze GJ., Jr Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989;338(6215):518–520. doi: 10.1038/338518a0. [DOI] [PubMed] [Google Scholar]
- 46.Hackeng TM, Sere KM, Tans G, Rosing J. Protein S stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. Proc Natl Acad Sci USA. 2006;103(9):3106–3111. doi: 10.1073/pnas.0504240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ndonwi M, Tuley EA, Broze GJ., Jr The Kunitz-3 domain of TFPI-alpha is required for protein S-dependent enhancement of factor Xa inhibition. Blood. 2010;116(8):1344–1351. doi: 10.1182/blood-2009-10-246686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood JP, Ellery PE, Maroney SA, Mast AE. Protein S is a cofactor for platelet and endothelial tissue factor pathway inhibitor-alpha but not for cell surface-associated tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol. 2014;34(1):169–176. doi: 10.1161/ATVBAHA.113.302655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood JP, Bunce MW, Maroney SA, Tracy PB, Camire RM, Mast AE. Tissue factor pathway inhibitor-alpha inhibits prothrombinase during the initiation of blood coagulation. Proc Natl Acad Sci USA. 2013;110(44):17838–17843. doi: 10.1073/pnas.1310444110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nordfang O, Valentin S, Beck TC, Hedner U. Inhibition of extrinsic pathway inhibitor shortens the coagulation time of normal plasma and of haemophilia plasma. Thromb Haemost. 1991;66(4):464–467. [PubMed] [Google Scholar]
- 51.Erhardtsen E, Ezban M, Madsen MT, Diness V, Glazer S, Hedner U, Nordfang O. Blocking of tissue factor pathway inhibitor (TFPI) shortens the bleeding time in rabbits with antibody induced haemophilia A. Blood Coagul Fibrinolysis. 1995;6(5):388–394. doi: 10.1097/00001721-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Maroney SA, Cooley BC, Ferrel JP, et al. Absence of hematopoietic tissue factor pathway inhibitor mitigates bleeding in mice with haemophilia. Proc Natl Acad Sci USA. 2012;109(10):3927–3931. doi: 10.1073/pnas.1119858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eichler H, Kaan Kavakli PAK, Knoebl P, Windyga J, Jiménez-Yuste V, Friedrich U, Chowdary HP, et al. Mechanistic modeling of the pharmacodynamic and pharmacokinetic relationship of tissue factor pathway inhibitor-neutralizing antibody (BAY 1093884) in Cynomolgus Monkeys. AAPS J. 2017;19(4):1186–1195. doi: 10.1208/s12248-017-0086-4. [DOI] [PubMed] [Google Scholar]
- 54.Gu JM, Zhao XY, Schwarz T, et al. Mechanistic modeling of the pharmacodynamic and pharmacokinetic relationship of tissue factor pathway inhibitor-neutralizing antibody (BAY 1093884) in Cynomolgus Monkeys. AAPS J. 2017;19(4):1186–1195. doi: 10.1208/s12248-017-0086-4. [DOI] [PubMed] [Google Scholar]
- 55.Paz P, Xie J, Aswad F. Antibody engineering of anti-tfpi bypass therapeutic BAY 1093884: isotype selection and sequence optimization. Blood. 2015;126(23):3496. [Google Scholar]
- 56.Rakhe S, Hett SP, Murphy JE, Pittman DD. An antibody to tissue factor pathway inhibitor (PF-06741086) in combination with recombinant factor VIIa increases hemostasis in haemophilia plasma without excessive thrombin generation. Blood. 2016;128(22):2566. [Google Scholar]
- 57.Lauritzen B, Hilden I. Concizumab promotes hemostasis via a TF-FVIIa dependent mechanism as shown in a rabbit haemophilia bleeding model. Blood. 2016;128(22):1401. doi: 10.1111/hae.13861. [DOI] [PubMed] [Google Scholar]
- 58.Waters EK, Sigh J, Friedrich U, Hilden I, Sorensen BB. Concizumab, an anti-tissue factor pathway inhibitor antibody, induces increased thrombin generation in plasma from haemophilia patients and healthy subjects measured by the thrombin generation assay. Haemophilia. 2017;23(5):769–776. doi: 10.1111/hae.13260. [DOI] [PubMed] [Google Scholar]
- 59.Lin JH. Dose-dependent pharmacokinetics: experimental observations and theoretical considerations. Biopharm Drug Dispos. 1994;15(1):1–31. doi: 10.1002/bdd.2510150102. [DOI] [PubMed] [Google Scholar]
- 60.Levy G. Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther. 1994;56(3):248–252. doi: 10.1038/clpt.1994.134. [DOI] [PubMed] [Google Scholar]
- 61.Mager DE. Target-mediated drug disposition and dynamics. Biochem Pharmacol. 2006;72(1):1–10. doi: 10.1016/j.bcp.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 62.Agerso H, Overgaard RV, Petersen MB, et al. Pharmacokinetics of an anti-TFPI monoclonal antibody (concizumab) blocking the TFPI interaction with the active site of FXa in Cynomolgus monkeys after iv and sc administration. Eur J Pharm Sci. 2014;56:65–69. doi: 10.1016/j.ejps.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Berckmans RJ, Nieuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85(4):639–646. doi: 10.1055/s-0037-1615646. [DOI] [PubMed] [Google Scholar]