Figure 2.
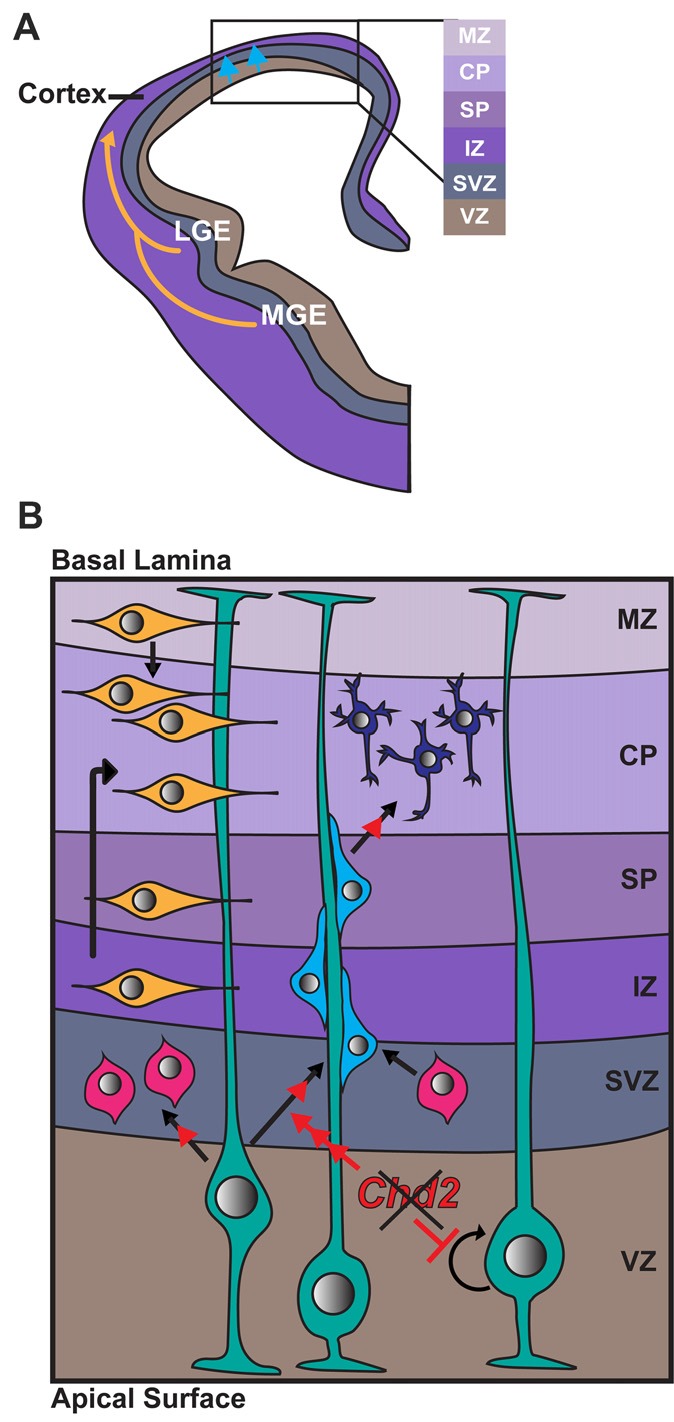
Role of CHD2 in differentiation of excitatory and inhibitory neurons. (A) A schematic representation of a coronal section of one half of an embryonic forebrain showing the subdivisions of the telencephalic proliferative zones: lateral ganglionic eminence (LGE) and medial ganglionic eminence (MGE). Excitatory neurons are born in the ventricular zone (VZ) of the cortex and migrate toward the brain surface (blue arrows). Most inhibitory interneurons of the cortex originate in the MGE and LGE, migrating tangentially to colonize the cortex (yellow arrows). Biallelic knockdown of CHD2 in vitro has been shown to impair interneuron development. In vivo, CHD2 loss may hypothetically result in fewer interneurons migrating from the MGE/LGE or immature interneurons in the forebrain (B) Following migration to the cortex, GABAergic inhibitory interneurons (yellow) follow either a superficial migratory stream through the cortical plate (CP) and marginal zone (MZ) or migrate through the deep layers of the subventricular (SZ) and intermediate zones (IZ). In early stages of development, radial glial cells (RGCs; green) divide symmetrically to produce two new RGCs and replenish the progenitor pool (represented by circular arrow). As development progresses, Pax6+ RGCs begin to divide asymmetrically in the VZ, giving rise to excitatory neurons (shades of blue) or Tbr2+ intermediate progenitors (IPs; pink). Chd2 is expressed primarily in RGCs in the VZ/SVZ and is not expressed in IPs. When Chd2 is disrupted, self-renewal of RGCs is diminished, there is an increase in the production of IPs, and more cells differentiate into glutamatergic neurons (red arrows; Anderson et al., 2001; Kriegstein and Noctor, 2004; Wu et al., 2011; Shen et al., 2015). CP, cortical plate; IZ, intermediate zone; LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; MZ, marginal zone; SP, subplate; SVZ, subventricular zone; VZ, ventricular zone.
