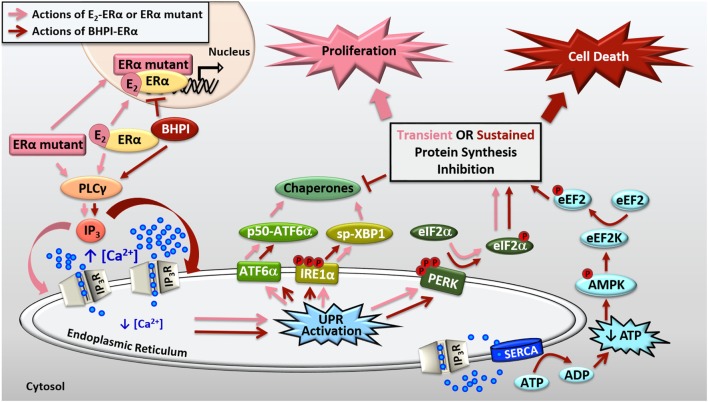
Figure 2.
Activation of the anticipatory unfolded protein response by estrogen receptor α (ERα). E2-ERα and constitutively active ERα mutants activate a mild and protective anticipatory unfolded protein response (UPR) and the non-competitive biomodulator BHPI binds ERα and induces hyperactivation of this pathway leading to cell death. ERα indirectly activates phospholipase C γ (PLCγ), resulting in cleavage of PIP2 to DAG (diacylglycerol) and IP3 (inositol triphosphate). E2-ERα and constitutively active ERα mutants cause moderate IP3 production, whereas BHPI causes significantly more production of IP3. The IP3 then binds to IP3 receptors (IP3Rs) in the endoplasmic reticulum (EnR) membrane, causing efflux of calcium from the lumen of the EnR into the cell body. E2-ERα and constitutively active ERα mutants cause moderate and transient release of calcium, resulting in weak and transient activation of all three arms of the UPR. Weak UPR activation results in very mild and transient inhibition of protein synthesis, production of molecular chaperones, and is critical for subsequent cell proliferation. BHPI-ERα induced hyperactivation of the UPR causes robust and sustained release of calcium from the EnR. This leads to robust PERK activation and rapid, sustained, and near-quantitative inhibition of protein synthesis. Although BHPI causes upregulation of chaperone mRNA, no protein is made, and the UPR-activating signal is never resolved. In an effort to re-establish cellular calcium homeostasis, ATP-dependent SERCA pumps in the EnR actively transport calcium back into the lumen of the EnR but since IP3Rs remain open, an ATP-depleting futile cycle ensues. Decreased cellular ATP and increased AMP activate AMPK, which along with calcium, activates Ca2+/calmodulin-dependent kinase, eukaryotic elongation factor 2 kinase (CAMKIII/eEF2K). eEF2K then phosphorylates eEF2, causing inhibition of protein synthesis at elongation. Ultimately, BHPI-ERα induced hyperactivation of the anticipatory UPR causes death of ERα positive endometrial and breast cancer cells.