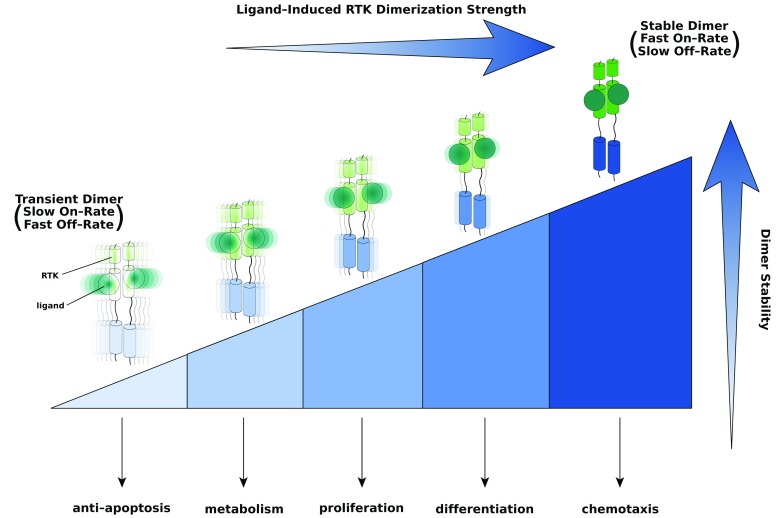
Figure 2. A unifying threshold model for receptor tyrosine kinase (RTK) signaling specificity and cell fate determination.
Different thresholds in dimer strength/stability are required to alter the transcriptional landscape and cellular fate. The strongest/most-stable dimer—at the right-hand end of the threshold spectrum (depicted by the shaded/sectioned triangle)—is able to perform any and all cell responses to its left. By contrast, the weakest/less-stable dimer—pictured at the left end of the spectrum—is capable only of eliciting a single response. The on- and off-rates of a prototypical ligand-induced RTK dimer (that is, dimer stability) are determined by the energetics of ligand–receptor, receptor–receptor, and other protein–protein binding events specific to a given RTK. Based on the model, therapeutically useful ligands can be engineered by fine-tuning receptor dimer stability through changes in the strengths of individual protein–protein interactions involved in dimerization. Intensity of color and sharpness of focus as well as oscillations of the dimer are used to emphasize differences in dimer strength/stability (that is, on- and off-rates) and rigidity.