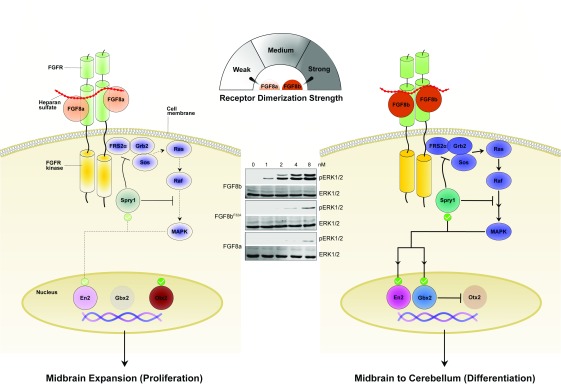
Figure 3. The “threshold model”, as exemplified by the FGF8-FGFR system, can explain disparities in transcriptional activity.
(Left) FGF8a dimerizes its receptor weakly, thus transducing a transient signal that can only weakly induce En2 and Spry1 expression, while totally failing to induce expression of Gbx2, the Otx2 suppressor. In the absence of Gbx2, the expression of Otx2 remains high. Under these conditions, proliferation of midbrain is encouraged. (Center) Immunoblots previously published in Huang et al. 33 (2017) showing a dose-dependent activation of the MAPK pathway (ERK1/2) by FGF8a, FGF8b, and FGF8b F32A in the BaF3 cell line. By introducing the F32A mutation or reducing the FGF8b concentration, the MAPK activation appears similar to FGF8a. Data are representative of three independent experiments. (Right) FGF8b dimerizes its receptor strongly, thus producing a robust and persistent signal that strongly induces En2, Spry1, and Gbx2 expression, which suppresses Otx2 expression. Under these settings, midbrain differentiates to cerebellum. Thus, quantitative differences in the stability of FGF-induced FGFR dimers translate into differences in the magnitude/duration of the intracellular signal, which in turn modify the transcriptional landscape and ultimately define the developmental response. Stronger receptor dimerization strength and higher signaling intensity are indicated by darker coloring. FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; MAPK, mitogen-activated protein kinase.