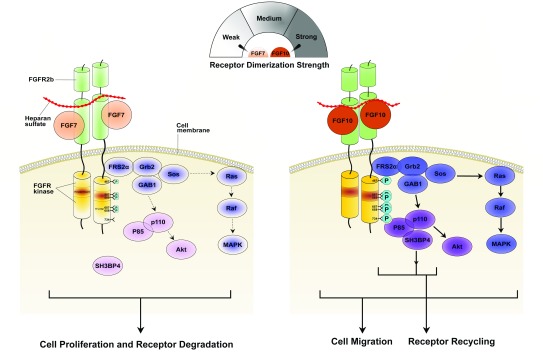
Figure 4. The “threshold model” can account for the activation of distinct pathways by FGF7 and FGF10 binding to FGFR2b.
(Left) Relative to FGF10, FGF7 exhibits poor affinities for both FGFR2b and the heparan sulfate (HS) co-receptor and hence forms a weaker dimer with FGFR2b. Consequently, FGF7-FGFR2b dimers are less efficient in promoting transphosphorylation of A-loop tyrosines (necessary for kinase activation) and altogether fail to phosphorylate Y734 (necessary for the recruitment of distinct substrates and receptor recycling). Weaker kinase activation corresponds with a transient and overall weaker activation of MAPK that is further exacerbated by the inability of FGFR2b to recycle to the cell surface. (Right) FGF10 dimerizes its receptor strongly, promoting robust phosphorylation of A-loop tyrosines so as to transduce a prolonged signal. Y734 also becomes phosphorylated, thus enabling the recruitment of the P85-SH3BP4 complex in order to elicit receptor recycling and cell migration. Stronger receptor dimerization strength and higher signaling intensity and phosphorylation levels are indicated by darker coloring. A-loop, activation loop; FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; MAPK, mitogen-activated protein kinase.