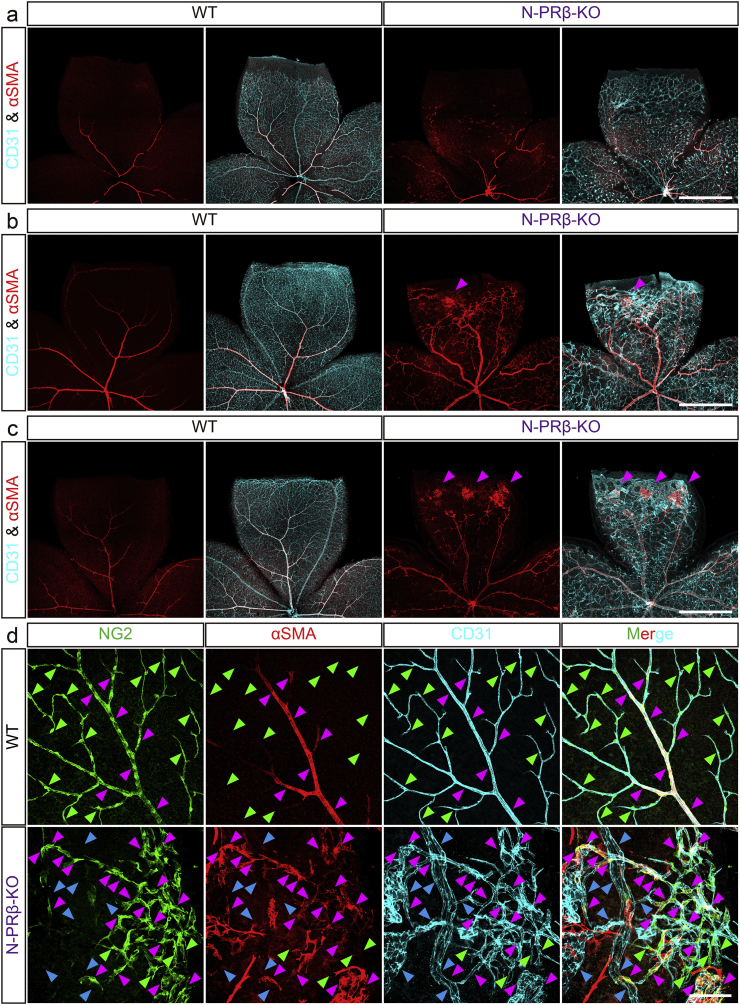
Fig. 3.
Foci of proliferative membrane and structural abnormality of blood vessels are observed in early stage of N-PRβ-KO mouse retina.
(a–c) Retinal angiogenesis of WT and N-PRβ-KO at 1 week (a), 2 weeks (b) and 4 weeks (c). CD31+ retinal vascular networks (cyan) develop toward the peripheral region. αSMA+ pericytes (red) that preferentially reside on the arteriole and venule are observed (a–c, left) in WT retinas. Owing to a loss of NG2+αSMA− microvascular pericytes, N-PRβ-KO retina at 1 week show relatively large-sized capillaries. Additionally, defects in the specification of arterioles and venules are often observed, and atypical migration of NG2+αSMA+ pericytes can be found (a, right). Blood vessel dilation and microvascular aneurisms are often observed at 2 to 4 weeks. At the same time points, foci of proliferative membrane constituted by αSMA+ pericytes (magenta-arrowheads, myofibroblast-like phenotype) are observed (b and c, right). (d) Multi-colour representative immunofluorescence images of WT (upper row) and N-PRβ-KO mice (bottom row) at 4 weeks. NG2+ pericytes (green), αSMA+ pericytes (red), CD31-positive blood vessels (cyan). In WT, whereas NG2+αSMA+ pericytes are preferentially observed on arterioles, venules and their primary branches (magenta-arrowheads), NG2+αSMA− pericytes are exclusively observed on the capillaries (green-arrowheads). In N-PRβ-KO, considerable NG2+αSMA+ pericytes are observed on microvasculature (magenta-arrowheads) instead of NG2+αSMA− pericytes (green-arrowheads). In addition, naked blood vessels are often observed (blue-arrowheads). Scale bar = 1 mm (a–c), 100 μm (d).