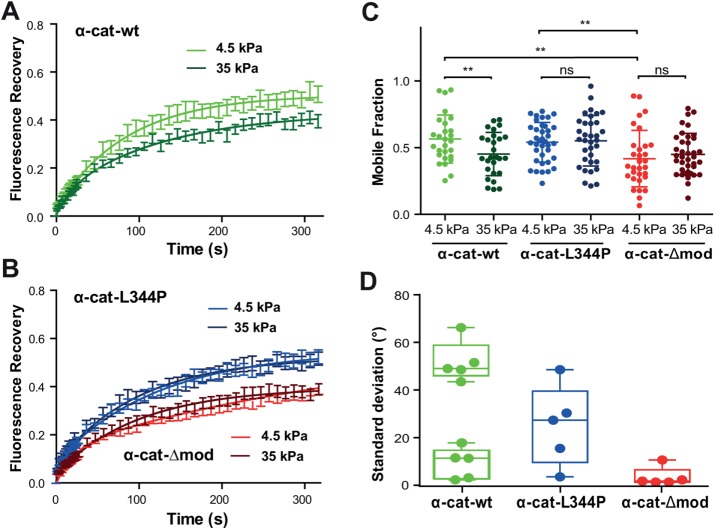
FIGURE 3:
Binding of α-catenin to vinculin is required for its tension-dependent stabilization at cell–cell contacts. (A, B) MDCK α-catenin–KD cells expressing GFP-tagged α-cat-wt (green), α-cat-L334P (blue), or α-cat-Δmod (red) were cultured for 24 h on 4.5 (light colors) or 35 kPa (dark colors) PPA gels before FRAP experiments were performed. Graphs represent mean GFP fluorescence recovery over time (±SEM, n = 50 out of three independent experiments for each condition) fitted with a one-term exponential equation. (C) Mobile fraction values (scatter dot plot, mean values ± SD) extracted from the fits of individual recovery curves considered in panels A and B. **, p < 0.01; ns, nonsignificant; two-way ANOVA test. Notice the nonsignificant differences in mobile fraction values observed for the mutant proteins on soft and stiff substrates, contrasting with the significant decrease in mobile fraction observed for the wt protein as a function of increasing substrate compliance. (D) Magnetocytometry applied on Ecad-Fc–coated bead doublets bound to the surface of MDCK cells expressing α-cat-wt, α-cat-L334P, and α-cat-Δmod mutants. The histogram reports the mean values of the SD of the bead fluctuation angles.