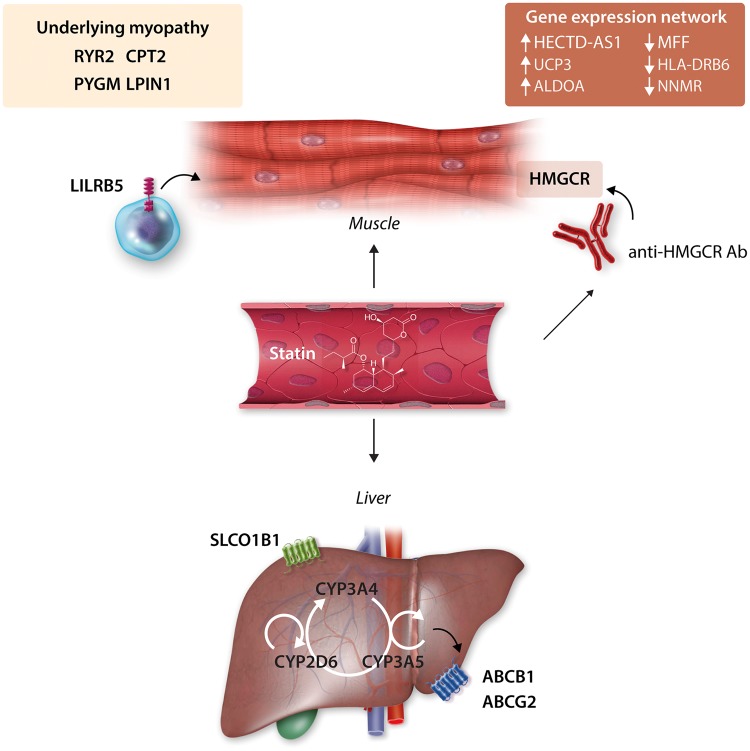
Figure 1.
Biological mechanisms of statin-associated muscle symptoms (SAMS) implicated by genetics. A loss-of-function variant in the SLCO1B1 gene, which encodes a hepatic statin transporter, results in increased plasma statin concentration and increases the risk of SAMS, particularly for simvastatin. Variation in cytochrome P450 metabolic enzymes including CYP3A4, CYP3A5, and CYP2D6 may also predispose to SAMS. Variation in transporters responsible for efflux of statins from hepatocytes including ABCB1 and ABCG2 are also linked to increased plasma concentration and risk of SAMS. At the level of the muscle, statin use may unmask underlying or pre-symptomatic myopathic conditions, for example, those caused by variation in the CPT2, PYGM, RYR2, and LPIN1 genes. The LILRB5 gene, a leucocyte receptor expressed predominantly in immune cells, has been implicated in SAMS, in particular carriers of the p.Asp247 allele. The mechanism is uncertain, but may involve immune-mediated repair of muscle injury. Patients with SAMS also display a specific gene-expression network in muscle that involves pathways related to cellular stress, repair, immune response, and protein catabolism. Finally, statin treatment in immunogenetically susceptible individuals (those with the DRB1*11: 01 allele) can lead to the production of anti-HMG CoA Reductase (anti-HMGCR) antibodies, which may have a direct pathogenic effect on muscle tissue.