Abstract
Background: The progresses made in stem cell therapy offer an innovative approach and exhibit great potential for the repair of damaged organs and tissues. This study was conducted with a view to find the mechanisms responsible for the effectiveness of bone marrow-derived mesenchymal stem cells (BM-MSCs) in the suppression of diabetes and experimentally-induced diabetic nephropathy.
Methods: To realize this objective, diabetic and diabetic nephropathy subject groups that underwent MSC treatment were studied through numerous biochemistry and molecular genetics analyses.
Results: The findings show that, relative to the control groups, the rats in the diabetic and diabetic nephropathy groups treated with stem cells infused with BM-MSCs showed a significant reversal in the levels of their insulin, glucose, heme-oxygenase-1 (HO-1) serum, and advanced glycation end product (AGEP). Moreover, BM-MSC therapy was also found to have a definite positive effect on the kidney functions. In addition, it also corresponded with a significant decrease in the availability of certain growth factors, namely the fibroblast growth factor (FGF), the platelet-derived growth factor (PDGF), and the transforming growth factor-β (TGF-β). BM-MSC treatment also improved the levels of expression of monocyte chemoatractant-1 (MCP-1) and interleukin-8 (IL-8) genes within kidney tissues. Lastly, the treatment recovered the organizational structure of the kidney and pancreas, a result demonstrated by a histopathological analysis. These results greatly coincide with those obtained through the biochemistry and molecular genetics analyses.
Conclusion: Treatment using BM-MSCs is determined to be definitely effective in cases of diabetes and diabetic nephropathy.
Keywords: Diabetes, diabetic nephropathy, growth factors, inflammatory mediators, oxidative stress markers, stem cells
Introduction
Diabetes mellitus is a complicated disease of the metabolism that is an ever-growing concern in today’s society, as it is predicted to affect an estimated 500 million people in the next 15 years.1 Moreover, diabetes has been linked to serious chronic microvascular and macrovascular issues and is associated with large likelihoods of further health issues and mortality. Both types of diabetes, 1 and 2, are serious and common health risks with several long-term negative effects, which can thus lead to ever-growing expense.2
Diabetic nephropathy (DN) is one of the many complications associated diabetes mellitus, a life-threatening condition that is the main cause of end-stage renal disease (ESRD) in first world countries. An estimated 30% of diabetes mellitus type-1 (T1D) patients are also afflicted with nephropathy, often needing to undergo kidney dialysis or kidney transplantation.3 Moreover, an estimated 30–40% of diabetes mellitus type-2 (T2D) patients are also afflicted with diabetes-induced kidney disease.4
DN caused by the interaction of haemodynamic and metabolic factors.5 In fact, one of the major factors in kidney damage due to high glucose levels is reactive oxygen species (ROS). Some of the distinguishing characteristics of this illness are proteinuria, degeneration of renal activity, and, from histopathology, mesangial expansion and, afterwards, glomerulosclerosis.3 In addition, some pathological changes associated with DN are renal hypertrophy, as well as extracellular matrix accumulation, which in turn causes albuminuria and kidney failure via tubule interstitial fibrosis.6
Therapy through the use of stem cells has proved to be very promising in the treatment of tissue and organ damage, such as damage in the kidney. These are undifferentiated cells that have the ability to both self-multiply and differentiate into other specialized cells.7 In particular, multipotent mesenchymal stromal cells (otherwise called mesenchymal stem cells or MSCs) are stem cells that are present in nearly every postnatal tissue and organ, although they are most commonly obtained from the bone marrow.8 Of the different types of stem cells, MSCs have numerous characteristics that make them suitable for medical uses, such as the capability to relocate to tissue damage areas, potent immunosuppression, and postinfusion safety.5
According to earlier researches, MSCs have the ability to differentiate into such cell types as lipocytes, epithelial cells, hepatocytes, neurons, vascular endothelial cells, and myocardiocytes. This gives them a possibly critical role in the future of curing serious human illnesses. Thus, researchers have highlighted the great potential of MSCs in the treatment of diabetes and its complications due to the stem cells’ capacity for multipotent differentiation, combined with their self-renewing and immune response regulation capabilities.9,10
The current study was designed to investigate the effectiveness of bone marrow-derived mesenchymal stem cells (BM-MSCs) in the alleviation of experimentally-induced diabetes and diabetic nephropathy.
Methodology
Preparation of BM-MSCs
Isolated samples were obtained from the tibias and femurs of 6–8-week-old albino rats. From these samples, (BM-MSCs) were obtained. These were then suspended in DMEM media, which contained 10% fetal bovine serum and penicillin/streptomycin as an antibiotic (Cat #s 41965–039, 26400–036, and 15140–122, respectively, from Life Technologies-Gibco, UK). The atmospheric state was adjusted at 5% carbon dioxide. Afterwards, an inverted microscope was used to conduct morphological characterization in order to confirm the identity of BM-MSC.
Characterization of BM-MSCs via flow cytometry
PE-conjugated CD44 and CD49 antibodies and FITC-conjugated CD45 antibody (from Miltenyi Biotec, Germany and Dako Co., Denmark, respectively) were used to perform flow cytometry analysis on the BM-MSCs. This was done to verify that the phenotype of the stem cells was retained after expanding in the cell culture.11
As the BM-MSCs were incubated, each of the antibodies was placed against the surface markers: for 4 min for the CD44 and CD90 antibodies and for 30 min for the CD45 antibody, all at 4°C. Afterwards, flow cytometry analysis was performed on the samples (Beckman Coulter Elite XL, Nyon, Switzerland).
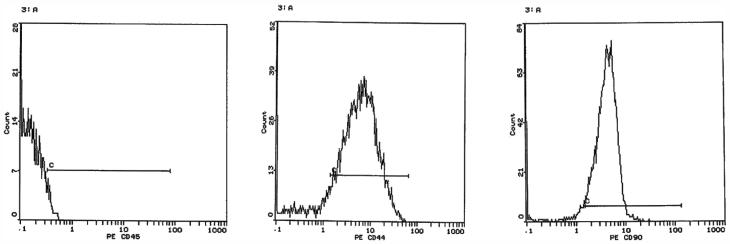
The results of the flow cytometry analysis are tabulated in Figure 1. Briefly, the analysis detected 76.88% of CD44, 85% of CD90, and a negligible 1.1% of CD45 in the isolated stem cells.
Figure 1.
Flow cytometry analysis of BM-MSCs after staining with FITC-conjugated CD44 and PE-conjugated CD105 antibodies: CD45 (1.1%), CD44 (76.88%) and CD90 (85%).
Treatment of animals
All the experiments that involved animal subjects and tissues were performed following the recommendations of the Guide for Care and Use of Laboratory Animals issued by the King Fahd Medical Research Center, Jeddah. Fifty female adult albino Wistar rats, with weights ranging from 180 g to 200 g, were involved in the current research.
Afterwards, the subjects were randomly designated into five groups of 10 rats each. Group (1) was the Control group, which were given a single intraperitoneal injection of citrate buffer (pH 4.5). Group (2) was the Diabetic group, which were given a single intraperitoneal injection of a mixture of 70 mg/kg streptozotocin (STZ) in 0.1 M citrate buffer.4 Group (3) was the diabetic nephorpathy (DN) group, which were given a single intraperitoneal injection of a mixture of 70 mg/kg streptozotocin in 0.1 M citrate buffer Thus, DN state was attained 6 weeks post-injection of STZ according to Balakumar et al.12 Group (4) was the diabetic group treated with MSCs (Diabetic + MSCs), which were given a single-dose intravenous treatment of 1.0 × 106 cells per subject. Lastly, Group (5) was the DN group treated with MSCs (DN + MSCs), which were given a single dose intravenous treatment of 1.0 × 106 cells per subject.
Peripheral blood from the tail vein was then tested to confirm diabetes induction (One Touch. Sure Step Meter, Life Scan, CA). The results were a blood glucose level of over 300 mg/dl post-injection of STZ. Meanwhile, creatnine and serum urea were measured to confirm DN induction (data not shown). Thus, DN state was attained 6 weeks post-injection of STZ. The same method was then used to measure fasting blood glucose levels 21 days after MSCs treatment. Afterwards, the subjects were allowed to fast for 12 h, after which 24-h urine samples were obtained. These were then processed in a refrigerated centrifuge at 4^ C at 1800×g for 20 min. The supernatants were separated, urea, microalbumin, and creatinine were analysed. Then, animals were anesthetized using diethyl ether, and blood samples were immediately withdrawn from the retro-orbital venous plexus. The subjects were then euthanized through dislocation of the cervix, after which each subject’s pancreas and kidneys were collected for dissection. The pancreas and one kidney per subject were stored in 10% formal saline to be analyzed histopathologically later, while one kidney was stored in liquid nitrogen for molecular genetics analyses.
Biochemical analyses
The blood glucose levels were obtained as in the previous section, while the serum insulin levels were obtained following the instructions by the manufacturer on the prepackaged kit (Immunospec Corporation, Canoga Park, CA). For each serum sample, the creatinine and urea levels were obtained using calorimetric methodology.13,14 Meanwhile, the determination of uric acid levels were based on methods outlined by Buchanan et al.15 protein levels based on methods outlined by Gornall et al.16 and albumin levels based on methods outlined by Doumas et al.17 On the other hand, urinary microalbumin was quantified through spectrophotometry, following the instructions by the manufacturer on the prepackaged kit (Reactivos GPL, Barcelona). Similarly, the (HO-1), (AGEP), (FGF), (PDGF), and (TGF-β) were quantified based on the instructions provided in the assay kit (Glory Science Co., Del Rio, TX). In each kit, the markers were estimated using a double antibody sandwich enzyme-linked immunosorbent assay.
Molecular genetic analysis
Semiquantitative RT-PCR
Trizol was used to extract the cellular RNA from the renal tissues of the subjects (Bioshop Canada Inc.). Spectrophotometry was then used to determine the concentration and purity of the RNA, at 260 nm and 280 nm. Only these samples with 1.8:2 ratios were utilized in the analyses. Afterwards, reverse transcription from RNA to cDNA was performed using a Revert Aid kit for first-strand cDNA synthesis (Fermentas Co., St. Leon-Rot, Germany).
The selection of the IL-8 gene, in the meantime, was based on the sequences published by Soliman el al.18 for the GAPDH gene those by Paul and Kundu,19 and for the MCP-1 gene those by Hajiasgharzadeh et al.20 These primer sequences for the genes are tabulated in Table 1.
Table 1.
Gene primer sequences used in PCR.
| Gene name | Gene primer sequences |
|---|---|
| GAPDH | F: 5′-CAAGGTCATCCATGACAACTTTG-3′ |
| R: 5′-GTCCACCACCCTGTTGCTGTAG-3′ | |
| Interleukin -8 (IL-8) | F: 5′-CTCCAGCCACACTCCAACAGA-3′ |
| R: 5′-CACCCTAACACAAAACAGAT-3′ | |
| Monocyte chemoattractantprotein-1 (MCP-1) | F: 5′-GGGCCTGTTGTTCACAGTTGC -3′ |
| R: 5′-GGGACACCTGCTGCTGGTGAT-3′ |
Histopathological analysis
The pancreas and kidney were prepared to hematoxylin and eosin staining according to Banchroft et al.21 Then, slides were observed using a light microscope (Olympus CH, Japan), on which images were captured (Sony DSCOW3, Japan) and photomicrograph calibrating was performed.22
Statistical analysis
Statistical analysis underwent one-way analysis of variance (ANOVA) using the program Statistical Package for the Social Sciences (SPSS v.17). Followed by least significant difference analysis for the comparison of significance in the different groups, with a significance level set at 5%.
Results
Biochemical analysis
Statistical analysis of the results showed a significant increase in blood glucose, coupled with a significant decrease in serum insulin levels in both diabetic and DN groups relative to the control group (p ≤ .05). Meanwhile, diabetic and DN groups treated with MSCs experienced significant suppression of blood glucose level, in association with significant elevation in serum insulin levels as compared to their untreated counterparts (p ≤ .05). The data are illustrated in Table 2.
Table 2.
Effect of MSCs infusion on serum glucose and insulin levels in diabetic and diabetic nephropathy-bearing rats (mean ± SE).
| Glucose (mg/dl) | Insulin (μU/mL) | |
|---|---|---|
| Control | 108.87 ± 3.22 | 11.51 ± 0.265 |
| Diabetic | 449 ± 20.86a | 7.79 ± 0.199a |
| DN | 526.37 ± 34.9a | 7.69 ± 0.319a |
| Diabetic + MSCs | 182.62 ± 16.2b | 10.28 ± 0.266b |
| DN + MSCs | 194.12 ± 14.52b | 9.6 ± 0.319b |
Significant change at p ≤ .05 in comparison with the control group.
Significant change at p ≤ .05 in comparison with diabetic and diabetic nephropathy-induced groups.
Table 3 represents the values of urea, creatinine, uric acid, total protein and albumin in serum of rats in the different studied groups. It could be observed that serum urea, creatinine, and uric acid revealed significant increase in diabetic and DN groups with respect to the control group (p ≤ .05), while serum total protein and albumin levels decreased significantly in these groups comparing to control group. On the other hand, diabetic and DN groups treated with MSCs showed significant reduction in serum urea, creatinine and uric acid levels as compared to the corresponding untreated groups (p ≤ .05). However, no significant change in serum albumin level and protein (p ≤ .05) were recorded in the treated groups relative to the corresponding untreated counterparts.
Table 3.
Effect of MSCs infusion on kidney functions and serum protein contents in diabetic and diabetic nephropathy-bearing rats (mean ± SE).
| Parameters groups | Urea mg/dl | Creatinine mg/dl | Uric acid mg/dl | Total protein mg/dl | Albumin mg/dl |
|---|---|---|---|---|---|
| Control | 17.82 ± 1.32 | 0.65 ± 0.0003 | 1.047 ± 0.005 | 8.67 ± 0.969 | 1.37 ± 0.0005 |
| Diabetic | 27.76 ± 0.758a | 0.945 ± 0.00024a | 2.26 ± 0.24a | 6.4 ± 0.27a | 0.977 ± 0.0004a |
| DN | 32.2 ± 1.93a | 0.976 ± 0.00027a | 3 ± 0.531a | 5.81 ± 0.194a | 0.993 ± 0.0006a |
| Diabetic + MSCs | 18.53 ± 2.58b | 0.862 ± 0.0003b | 1.107 ± 0.0008b | 7.87 ± 0.119b | 1.11 ± 0.00067 |
| DN + MSCs | 20.38 ± 2.29b | 0.887 ± 0.0036b | 1.29 ± 0.1407b | 7.38 ± 0.207b | 1.042 ± 0.00043 |
Significant change at p ≤ .05 in comparison with the control group.
Significant change at p ≤ .05 in comparison with diabetic and diabetic nephropathy-induced groups.
The data represented in Table 4 showed the concentration of urinary urea, creatinine, and microalbumin in the different studied groups. It could be seen that urinary urea, creatinine, and microalbumin concentrations revealed significant increase in diabetic and DN groups as compared to the control group (p ≤ .05). MSCs treatment produced significant regression in the urinary concentrations of urea, creatnine and microalbumin in the treated groups compared to the corresponding untreated groups (p ≤ .05).
Table 4.
Effect of MSCs infusion on urinary urea, creatinine and microlalbumin concentrations in diabetic and diabetic nephropathy-bearing rats (mean ± SE).
| Urea mg/dL | Creatinine mg/dL | Microalbumin mg/L | |
|---|---|---|---|
| Control | 664.66 ± 40.7 | 6.9 ± 0.188 | 3.05 ± 0.0076 |
| Diabetic | 1166.33 ± 27.46a | 7.8 ± 0.0198a | 3.95 ± 0.14a |
| DN | 1913.33 ± 50.25a | 9.95 ± 0.507a | 5.26 ± 0.009a |
| Diabetic + MSCs | 981.5 ± 12.96b | 7.183 ± 0.313b | 3.9 ± 0.0096b |
| DN + MSCs | 1014 ± 27.2b | 7.516 ± 0.212b | 3.83 ± 0.128b |
Significant change at p ≤ .05 in comparison with the control group.
Significant change at p ≤ .05 in comparison with diabetic and diabetic nephropathy-induced groups.
The results of serum (AGEP) level and (HO-1) activity are illustrated in Table 5. It is clear from the data that serum (AGEs) level showed insignificant increase in both diabetic and DN groups as compared to the control group (p ≤ .05). Meanwhile, serum (HO-1) activity revealed significant decrease in these groups relative to the control group (p ≤ .05). On the other hand, diabetic and DN groups treated with MSCs experienced insignificant regression in serum (AGEs) level (p ≤ .05) paralleled by significant increase in serum (HO-1) activity when compared with the corresponding untreated groups (p ≤ .05).
Table 5.
Effect of MSCs infusion on serum advanced glycation end products (AGEs) level and heme oxygenase-1 (HO-1) activity in diabetic and diabetic nephropathy-bearing rats (mean ± SE).
| AGEs(ng/L) | HO-1(ng/L) | |
|---|---|---|
| Control | 208.25 ± 2.23 | 1541 ± 67.75 |
| Diabetic | 261.5 ± 6.7 | 1131.37 ± 13.8a |
| DN | 288.25 ± 9.18 | 1100.37 ± 19.65a |
| Diabetic + MSCs | 240 ± 12.95 | 1245.37 ± 16.36b |
| DN + MSCs | 222.12 ± 7.5 | 1283 ± 16.15b |
Significant change at p ≤ .05 in comparison with the control group.
Significant change at p ≤ .05 in comparison with diabetic-induced group.
Regarding the growth factor data in Table 6, significant increase in serum (TGF-β), f (FGF-2), and (PDGF) in diabetic and DN groups compared to the control group (p ≤ .05). Meanwhile, the groups treated with MSCs showed significant depletion in these markers in comparison with the corresponding untreated groups (p ≤ .05).
Table 6.
Effect of MSCs infusion on serum growth factors (TGF-β, FGF-2, and PDGF) levels in diabetic and diabetic nephropathy-bearing rats (mean ± SE).
| TGF-β (ng/L) | FGF-2(ng/L) | PDGF(ng/L) | |
|---|---|---|---|
| Control | 30.51 ± 1.68 | 19.88 ± 2.799 | 1066.13 ± 22.52 |
| Diabetic | 47 ± 1.76a | 30.7 ± 0.494a | 1195.62 ± 2.013a |
| DN | 56.6 ± 1.91a | 36.27 ± 1.55a | 1253.83 ± 17.48a |
| Diabetic + MSCs | 35.93 ± 1.471b | 27.6 ± 0.863b | 1124.37 ± 13.4b |
| DN + MSCs | 36.16 ± 1.4b | 29.53 ± 0.872b | 1121.75 ± 22.38b |
Significant change at p ≤ .05 in comparison with the control group.
Significant change at p ≤ .05 in comparison with diabetic and diabetic nephropathy-induced groups.
Molecular genetic analysis
Molecular genetics analysis showed significant up-regulation of renal IL-8 and MCP-1 gene expression level in diabetic and DN groups with respect to the control group (p ≤ .05). Diabetic and DN groups treated with MSCs revealed significant down-regulation in the gene expression levels of IL-8 and MCP-1 when compared with the untreated counterparts (p ≤ .05). These results are shown in Table 7 and Figure 2.
Table 7.
Effect of MSCs infusion on iinterlukin-8 (IL-8) and monocyte chemoatractant-1(MCP-1) gene expression level in kidney tissue of diabetic and diabetic nephropathy-bearing rats (mean ± SE).
| IL-8 | MCP-1 | |
|---|---|---|
| Control | 0.368 ± 0.000598 | 0.287 ± 0.00197 |
| Diabetic | 1.055 ± 0.00451a | 0.097 ± 0.00491a |
| DN | 1.176 ± 0.00622a | 1.108 ± 0.00578a |
| Diabetic + MSCs | 0.719 ± 0.00228b | 0.48 ± 0.00427b |
| DN + MSCs | 0.324 ± 0.00416b | 0.348 ± 0.00311b |
Significant change at p ≤ .05 in comparison with the control group.
Significant change at p ≤ .05 in comparison with diabetic and diabetic nephropathy-induced groups.
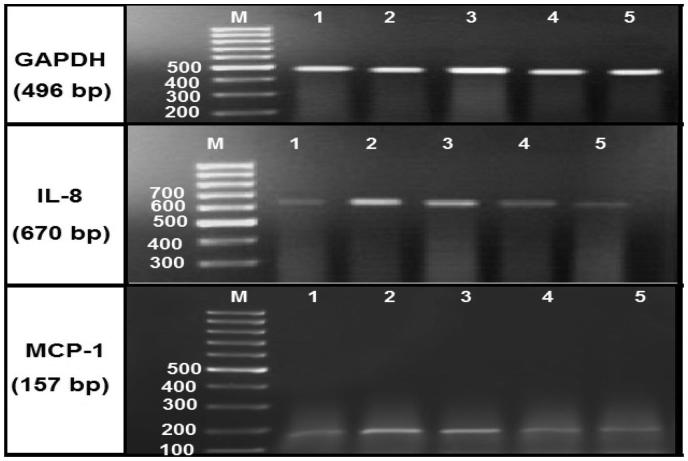
Figure 2.
Agarose gel electrophoresis showing GAPDH, IL-8 and MCP-1 mRNA expression in the kidney tissues by RT-PCR analysis. Lane (1): represents the control group and lane (2): represents diabetic group. Lane (3): represents diabetic nephropathy group, and lanes (4): represents diabetic group treated with MSCs, whereas lanes (5): represents diabetic nephropathy group treated with MSCs. Lane M: represents DNA ladder (100 bp).
Histopathological results
Pancreatic and kidney histopathological results are illustrated in Figures (3) and (4), respectively.
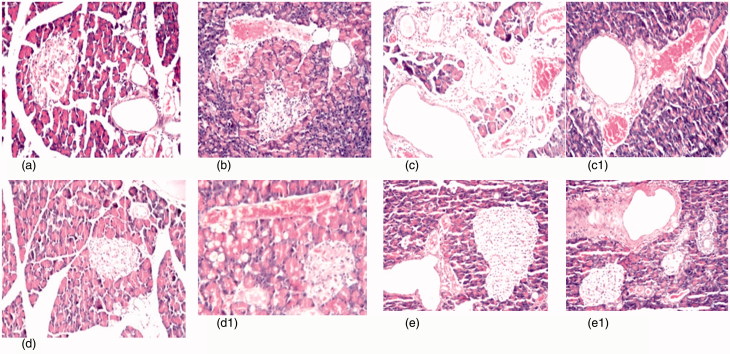
Figure 3.
(a) Photomicrograph of pancreas tissue section of control rats showing no histopathological alteration and the normal histological structure of the islands of Langerhans cells as endocrine portion as well as the acini and duct system as exocrine portion (H&Ex40). (b) Photomicrograph of pancreatic tissue section of diabetic rats showing atrophy in most of the islands of Langerhans cells with pyknosis of their nuclei and congestion in the blood vessels (H&Ex40). (c) Photomicrograph of pancreatic tissue section of diabetic nephroapathy rats showing edema with few inflammatory cells infiltration in the interlobular stroma (H&Ex40). (c1) It shows cystic dilatation in the ducts associated with congestion in the blood vessels (H&Ex40). (d, d1) Photomicrograph of pancreatic tissue section of diabetic rats treated with MSCs showing exocrine acini and fatty change in the lining epithelium with congestion in the blood vessels (H&Ex40). (e, e1) Photomicrograph of pancreatic tissue section of diabetic nephroapathy rats treated with MSCs showing no histopathological alteration in the islands of Langerhans cells, while the ducts showing periductal fibrosis.
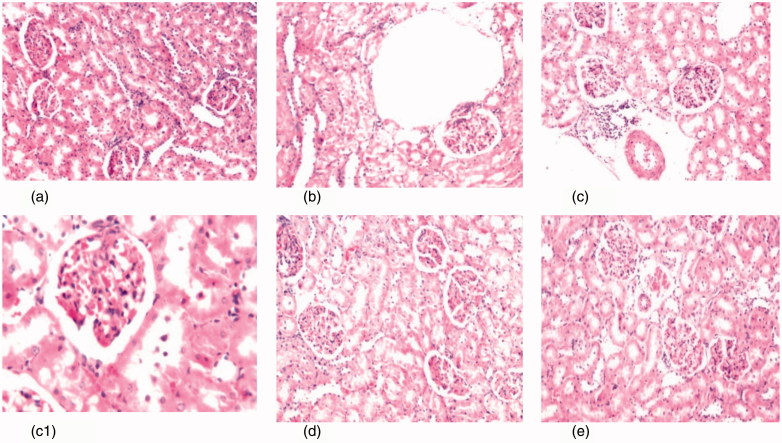
Figure 4.
(a) Photomicrograph of kidney tissue section of control rats showing no histopathological alteration and the normal histological structure of the glomeruli and tubules at the cortex are observed (H&Ex40). (b) Photomicrograph of kidney tissue section of diabetic rats showing degenerative changes and nephrosis in the tubular lining epithelium (H&Ex40). (c, c1) Photomicrograph of kidney tissue section of diabetic nephroapathy rats showing degenerative changes and nephrosis in the tubular lining epithelium associated with perivascular inflammatory cells aggregation surrounding the congested blood vessels (H&Ex40) and c1 (H&Ex80). (d) Photomicrograph of kidney tissue section of diabetic rats treated with MSCs showing congestion in the tufts of the glomeruli (H&Ex40). (e) Photomicrograph of kidney tissue section of diabetic nephroapathy rats treated with MSCs showing congestion in the glomeruli and blood vessels (H&Ex40).
Discussion
There has recently been increasing interest directed at stem cell therapy as a new innovation in the treatment of long-term illnesses, as well as the related complications. The central objective of the current study was to determine the effectiveness of bone marrow-derived mesenchymal stem cells on the amelioration of experimentally induced diabetes and diabetic nephropathy.
The results of the experiments indicate significantly increased blood glucose levels, coupled with significantly reduced serum insulin levels in both the diabetic and diabetic nephropathy groups. According to Kim et al.,23 the majority of diabetes loss pathology can be traced to the decrease and/or dysfunction of beta-cells.2 Different reasons have been put forward for this decrease, such as oxidative stress, endoplasmic reticulum stress, and amyloid formation toxicity.24 It must also be noted that, as diabetes cases progress, β-cell mass levels decrease and glucose levels increase. As the environment becomes hyperglycemic, the β-cells functions become impaired, especially the secretion of insulin. This effect, which is reversible, is determined to be due to glucose toxicity.10
In cases of diabetes and diabetic nephropathy induced by STZ injection, changes in the glomerulus functions have been noted, such as microalnuminuria; higher urea, creatine, and uric acid levels; and lower protein and albumin levels. The results of the present experiment coincide with those in the study conducted by Yamamoto et al.,3 in which they determined that initial phases of nephropathy is characterized by increases in proteinuria, nephromegaly.
Microalbuminaria is a critical marker in kidney damage, especially in cases of diabetes.25 Microalbuminaria results in high blood pressure, uremia, albuminuria, progressively declining glomerular filtration rate (GFR), and end-stage renal disease (ESRD).26
According to a study by Feig et al.,27 a high concentration of uric acid has been associated with (T2D). Resistance to insulin causes acidic urine. When the pH is low in urine, uric acid stones tend to form. In addition, excessive levels of blood uric acid are another complication of T2D, this leads to pro-inflammatory changes in the endocrine balance in the adipose tissue and vascular smooth muscle cells.28
The results of the present experiment revealed insignificant increase in serum AGEP levels in both diabetic and diabetic nephropathy groups. On the other hand, there was a significant decline in the activity of serum HO-1. In diabetes mellitus, extended hyperglycemia causes accumulation of AGEPs in the blood and different tissue.29
One of the expected ways of AGEPS to aggravate diabetes is cross-linking critical molecules in the ECM basement membrane, which disrupts the normal cell system. Another is through contact with their receptors (RAGE) on a cellular surface.30 Furthermore, glycation leads to an increase in the production of types 3, 5, and 6 collagen, fibronectin, and laminin in the ECM, probably through the up-regulation of TGF-β intermediates.31
Heme oxygenase (HO) is a rate-limiting enzyme that catalyzed breakdown of heme yields cytoprotective products including bilirubin, ferreittin, and carbon monoxide.32. The up-regulation of renal HO-1 expression prevents the development of metabolic syndrome and improves both vascular and renal function. Also, a study by Pitlovanciv et al.,32 showed that HO-1 induction is protective in many acute and chronic renal insults. These findings supported those of Elmarakby et al.33
Regarding TGF-β, PDGF, and FGF, the present data showed significant increase in the circulating levels of these indicators in diabetic and diabetic nephropathy groups. The present results were supported by Yener et al.34 Previous studies have shown the important role of hyperglycemia and hyperinsulinemia in increasing TGF-β expression through different pathways.35 In experimental diabetic mice, macrophage accumulation and activation as well as prolonged hyperglycemia has been associated with glomerular immune complex deposition, increased chemokine production, and progressive fibrosis. TGF-β has been recognized as a central player in the diabetic nephropathy as it is being involved in the development of glomerulosclerosis and interstitial fibrosis, as observed in the course of end-stage renal disease.26 Increasing evidence suggests that growth factors may contribute to the initiation and progressive fibrosis of diabetic nephropathy.36 Particularly, the roles of TGF-β and connective tissue growth factor have been described in diabetic nephropathy.44 In addition to its proliferative actions, PDGF also induces extracellular matrix formation in vitro with infusion and transfection studies confirming the pro-sclerotic effects of PDGF in nondiabetic renal disease in the in vivo setting.36
Expression of FGF-2 has been found to be significantly up-regulated in diabetic and diabetic nephropathy compared with the control with a good correlation to the degree of kidney injury.37 Fibroblasts cultivated in high glucose displayed increased FGF-2 mRNA as well as protein synthesis and secretion compared with normal glucose.38
Regarding molecular genetics results, significant up-regulation in gene expression levels of IL-8 and MCP-1 in kidney tissue were recorded in both diabetic and diabetic nephropathy groups. Aydin et al.39 found that IL-8 serum level tend to increase in the diabetic group. The increased IL-8 serum level in this group was attributed to the increased oxidative stress and inflammation due to hyperglycemia. Also, Khajehdehi et al.40 stated significant elevation in TGF-β and IL-8 serum levels in diabetic nephropathy bearing rats. These findings are in great agreement with the present results.
Hyperglycemia induces MCP-1 production in vascular endothelial cells and has been implicated as a causal factor in the facilitation of vascular complications in diabetes.41 It has been documented that high glucose level stimulates the expression of MCP-1 and the formation of (ROS), which may up-regulate MCP-1 expression by activation of the transcription factor NF-B.42 Morii et al.,43 suggested that heavy protenuria aggravates renal tubular damage and accelerates the disease progression in diabetic nephropathy by increasing the MCP-1 expression in renal tubule. These data are consistent with our results.
Considering the efficacy of stem cell therapy, the current results showed significant ameliorative effect on all the biochemical markers as well as histopathological features of pancreas and kidney of diabetic and DN bearing rats. In accordance with current results, an early study reported that transplantation of c-kit expressing BMSCs resulted in localization to ductal and islet structures and enhanced insulin secretion. Although occurring in low frequency, these results suggest that BMSCs have the ability to differentiate into β-cells.44 Xi and Bu,2 found that autologous MSCs transplantation in a rat model of (T2D) resulted in enhanced insulin secretion, increased islet numbers in pancreas and ameliorated insulin sensitivity, suggesting functional effects of MSCs on insulin target tissues which lead to the reduction of blood glucose level as shown in the present work.
Bone marrow–derived stem cells contribute to cell turnover and repair in various tissue types, including the kidneys. MSCs are attractive candidates for renal repair, because nephrons are of mesenchymal origin.45
The present study indicated that MSCs treatment attenuated diabetic nephropathy by significantly lowering serum urea and creatinine levels in diabetic rats. This could be explained as there was increased clearance of blood urea and creatinine by the kidney. This means that rats treated with MSCs had less renal injury. In consistent with Wang et al.,4 and Pitlovanciv et al.,32 stated that MSCs treatment in diabetic rats could maintain plasma levels of creatinine and urea near the control values suggesting the beneficial role of MSCs either directly or indirectly in providing protection against DN. Moreover, Wang et al.,46 proved that MSCs decrease albuminuria and preserve normal renal histology in diabetic mice. In addition, Rookmaaker et al.,47 declared that bone-marrow derived cells may home to injured glomerular endothelium, differentiate into endothelial cells, and participate in regeneration of the glomerular microvasculature.
The pharmacological induction of HO-1 has also been shown to exert a protective effect on renal function in animal models. MSCs have the capacity to repair renal injury, accelerate tubular proliferation and improve renal function as well as up-regulate HO-1 expression and increase HO-1 activity.34 These findings are in good agreement with our results.
MSCs treatment showed significant regression of TGF-β, FGF, and PDGF serum levels as shown in the present results. It could be deduced that the mechanisms that mediate the protective effects of MSCs most probably, paracrine. Duffield et al.,49 documented that BM-MSCs contribute in modulating cytokine environment of the damaged tissues, resulting in their functional repair. Moreover, bone marrow–derived stem cells seemed to contribute in regenerating renal tubular cell populations.48
In harmony with our findings, the study of Sho-Sho et al.,49 revealed that the expressions of MCP-1, IL-1, and IL-6 were significantly down-regulated by MSCs treatment in kidney tissue. MSCs treatment ameliorates DN via inhibition of MCP-1 expression by secreting growth factors, thus reducing macrophages infiltration, down regulating IL-8, IL-6, and TNF-α expression in renal tissue of diabetic rats.
Histopathological examination of pancreatic and kidney tissue samples of diabetes and DN bearing rats before and after MSCs infusion supported our biochemical results.
The favorable impact of BMSCs treatment was evidenced by recovery of kidney functions, glucose, insulin, HO-1, and AGEs in diabetic and diabetic nephropathy bearing rats. Also, BM-MSCs revealed a strong ability to modulate growth factors and down-regulate MCP-1 and IL-8 gene expression in kidney tissues. Taken collectively, the present work would indicate that the effect of cell therapy in diabetes and diabetic complications was rather agreeable.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Abu-Rmeileh NM, Husseini A, Capewell S, O’Flaherty M.. Preventing type 2 diabetes among Palestinians: Comparing five future policy scenarios. BMJ Open. 2013;3:e003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xi Y, Bu S.. Stem cells therapy in diabetes mellitus. J Stem Cell Res Ther. 2014;4:5 http://dx.doi.org/10.4172/2157-7633.1000199. [Google Scholar]
- 3.Yamamoto Y, Kato I, Doi T, et al Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest. 2001;108:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang G, Lu X, Li W, Zhao X, Zhang C.. Protective effects of luteolin on diabetic nephropathyin STZ-induced diabetic rats. Evid Based Complement Alternat Med. 2011;2011:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel Aziz MT, Wassef MA, et al The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. Diabetol Metab Syndr. 2014;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forbes JM, Fukami K, Cooper ME.. Diabetic nephropathy: Where hemodynamics meets metabolism. Exp Clin Endocrinol Diabetes. 2007;115:69–84. [DOI] [PubMed] [Google Scholar]
- 7.Weissman IL.Stem cells: Units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. [DOI] [PubMed] [Google Scholar]
- 8.Porada CD, Zanjani ED, Almeida-Porad G.. Adult mesenchymal stem cells: A pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006;1:365–369. [DOI] [PubMed] [Google Scholar]
- 9.Volarevic V, Arsnijevic N, Lukic M, Stojkovic M. Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weir G, Weder C, Weir SB.. Stem cell approaches for diabetes: Towards beta cell replacement. Genome Med. 2011;3:61 http://genomemedicine.com/content/3/9/61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodbury D, Schwarz EJ, Prockop DJ, Black IB.. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000; 61:364–370. [DOI] [PubMed] [Google Scholar]
- 12.Balakumar P, Chakkarwar VA, Kumar V, Jain A, Reddy J, Singh M.. Experimental models for nephropathy. J Renin Angiotensin Aldosterone Syst. 2008; 9:4. [DOI] [PubMed] [Google Scholar]
- 13.Larsen K. Creatinine assay by a reaction-kinetic principle. Clin Chim Acta. 1972;41:209–217. [DOI] [PubMed] [Google Scholar]
- 14.Orsonneau JL, Massoubre C, Cabanes M. Lustenberger. Simple and sensitive determination of urea in serum and urine. Clin Chem. 1992; 38:619–623. [PubMed] [Google Scholar]
- 15.Buchanan MJ, Isdale IC, Rose BS. Serum uric acid estimation chemical and enzymatic methods compared. Ann Rheum Dis. 1965;24:285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gornall AG, Bardawill CJ, David M.. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:201–207. [PubMed] [Google Scholar]
- 17.Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971;31:87–96. [DOI] [PubMed] [Google Scholar]
- 18.Soliman MM, Nassan MA, Ismail TA.. Immunohistochemical and molecular study on the protective effect of curcumin against hepatictoxicity induced by paracetamol in Wistar rats. BMC Complement Altern Med. 2014;14:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul S, Kundu R.. Antiproliferative activity of methanolic extracts from two green algae, Enteromorpha intestinalis and Rizoclonium riparium on HeLa cells. DARU J Pharm Sci. 2013;21:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hajiasgharzadeh K, Tavangar SM, Javan M, Dehpour AR, Mani AR.. Does hepatic vagus nerve modulate the progression of biliary fibrosis in rats? Auton Neurosci. 2014;185:67–75. [DOI] [PubMed] [Google Scholar]
- 21.Banchroft JD, Stevens A, Turner DR.. Theory and Practice of Histological Techniques 4th ed.New York, London, San Francisco, Tokyo: Churchill Livingstone; 1996. [Google Scholar]
- 22.Abramoff M.Image processing with imageJ. Biophotonics International. Imaging Software. 2004;11:36–42. [Google Scholar]
- 23.Kim JJ, Kido Y, Scherer PE, White MF, Accili D.. Analysis of compensatory beta-cell response in mice with combined mutations of Insr and Irs2. Am J Physiol Endocrinol Metab. 2007;292:E1694–E1701. [DOI] [PubMed] [Google Scholar]
- 24.Weir GC, Marselli L, Marchetti P, Katsuta H, Jung MH, Bonner-Weir S.. Towards better understanding of the contributions of overwork and glucotoxicity to the beta-cell inadequacy of type 2 diabetes. Diabetes Obes Metab. 2009;11(Suppl4):82–90. [DOI] [PubMed] [Google Scholar]
- 25.Satchell S, Tooke J.. What is the mechanism of microalbuminuria in diabetes: a role for the glomerular endothelium? Diabetologia. 2008;51:714–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes KB, Rodrigues KF, Fernandes AP.. The role of transforming growth factor-beta in diabetic nephropathy. Int J Med Genetics. 2014;2014:6 http://dx.doi.org/10.1155/2014/180270. [Google Scholar]
- 27.Feig DI, Kang D, Johnson RJ.. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amartey AA, Nsiah K, Mensa FO.. Plasma levels of uric acid, urea and creatinine in diabetics who visit the clinical analysis laboratory (CAn-Lab) at Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. J Clin Diagn Res. 2015;9:BC05–BC09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stitt AW, Li YM, Gardiner TA, Bucala R, Archar DB, Vlassara H. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats . Am J Pathol. 1997;150:523–531. [PMC free article] [PubMed] [Google Scholar]
- 30.Kass DA, Shapiro EP, Kawaguchi M, et al Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. [DOI] [PubMed] [Google Scholar]
- 31.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. [DOI] [PubMed] [Google Scholar]
- 32.Pitlovanciv E, Fernandes GS, Teixeira LC, et al Hemeoxygenase 1 improves glucose metabolism and kidney histological alteration in diabetic rats. Diabetol Metab Syndr. 2013;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elmarakby AA, Faulkner J, Baban B, Saleh MA, Sullivan JC.. Induction of hemeoxygenase-1 reduces glomerular injury and apoptosis in diabetic spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2012; 302:F791–F800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yener S, Comlekci A, Akinci B, et al Serum transforming growth factor-beta 1 levels innormoalbuminuric and normotensive patients with type 2 diabetes. Effect of metformin and rosiglitazone. Hormones. 2008; 7:70–76. [DOI] [PubMed] [Google Scholar]
- 35.Yener S, Demir T, Akinci B, et al Transforming growth factor-beta 1 levels in women with prior history of gestational diabetes mellitus. Diabetes Res Clin Pract. 2007;76:193–198. [DOI] [PubMed] [Google Scholar]
- 36.Langham RG, Kelly DJ, Maguire J, Dowling JP, Gilbert RE, Thomson NM.. Over-expression of platelet-derived growth factor in human diabetic nephropathy. Nephrol Dial Transplant. 2003;18:1392–1396. [DOI] [PubMed] [Google Scholar]
- 37.Vasko R, Koziolek M, Ikehata M, et al Role of basic fibroblast growth factor (FGF-2) in diabetic nephropathy and mechanisms of its induction by hyperglycemia in human renal fibroblasts. Am J Physiol Renal Physiol. 2009;296:F1452–F1463. [DOI] [PubMed] [Google Scholar]
- 38.Strutz F, Zeisberg M, Ziyadeh FN, et al Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61:1714–1728. [DOI] [PubMed] [Google Scholar]
- 39.Aydin M, Ozkok E, Ozturk O, et al Relationship between interleukin-8 and the oxidant-antioxidant system in end-stage renal failure patients. Exp Clin Transplant. 2007;5:610–613. [PubMed] [Google Scholar]
- 40.Khajehdehi P, Pakfetrat M, Javidnia K, et al Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: A randomized, double-blind and placebo-controlled study. Scand J Urol Nephrol. 2011;45:365–370. [DOI] [PubMed] [Google Scholar]
- 41.Jeon HJ, Choi HJ, Park BH, Lee YH, Oh T.. Association of monocyte chemoattractant protein-1 (MCP-1) 2518A/G polymorphism with proliferative diabetic retinopathy in Korean type 2 diabetes. Yonsei Med J. 2013;54:621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiarelli F, Cipollone F, Mohn A.. Circulating monocyte chemoattractant protein-1 and early development of nephropathy in type 1 diabetes. Diabetes Care. 2002;25:1829–1834. [DOI] [PubMed] [Google Scholar]
- 43.Morii T, Fujita H, Narita T, et al Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J Diabetes Complications. 2003;17:11–15. [DOI] [PubMed] [Google Scholar]
- 44.Antonello P.Mesenchymal stem cells for the treatment of diabetes. Diabetes. 2012;61:1355–1356. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anglani F, Forino M, Prete DD, Tosetto E, Torregrossa R, Angelo AD.. In search of adult renal stem cells. J Cell Mol Med. 2004;8:474–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S, Li Y, Zhao J, Zhang J, Huang Y.. Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. Biol Blood Marrow Transplant. 2013;19:538–546. [DOI] [PubMed] [Google Scholar]
- 47.Rookmaaker MB, Verhaar MC, de Boer H, et al Met-RANTES reduces endothelial progenitor cell homin to activated (glomular) endothelium in vitro and in vivo. Am J Physiol Renal Hysiol. 2007;293:F624–F630. [DOI] [PubMed] [Google Scholar]
- 48.Duffield JS, Bonvenetre JV.. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int. 2005;68:1956–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sha-Sha L, Liu G, Wang J, et al Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting macrophage infiltration. Int Immunopharmacol. 2013;17:275–282. [DOI] [PubMed] [Google Scholar]