Figure 2.
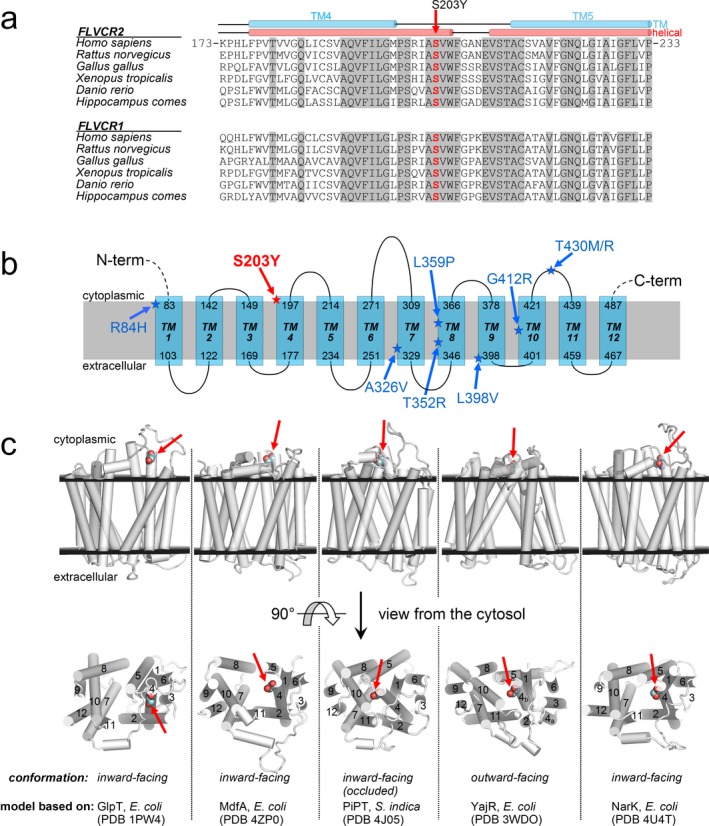
(a) Multiple sequence alignment of FLVCR2 protein (and the FLVCR1 paralogue) among species around the sites of the p.Ser203Tyr mutation (invariant residues are grayed). (b) Scheme of predicted membrane protein topology of FLVCR2. In red, the amino acid change identified in the present family. In blue, the amino acid change identified to date in Fowler syndrome. (c) Homology models of FLVCR2 (residues 86–491) based on structurally characterized MFS transporters. In the upper row of structures (viewed from the membrane side), the extracellular and cytoplasmic membrane layers (black planes) are positioned according to the predicted TM topology. The lower row represents the above structures rotated by 90 degree and viewed from the cytosol (TM regions 1–12 are labeled). Ser203 (site of the p.Ser203Tyr mutation) is shown as colored spheres and it is marked by red arrows. Substrates are transported across the membrane through the central part of the protein along the direction perpendicular to the figure plane of the cytosol view
