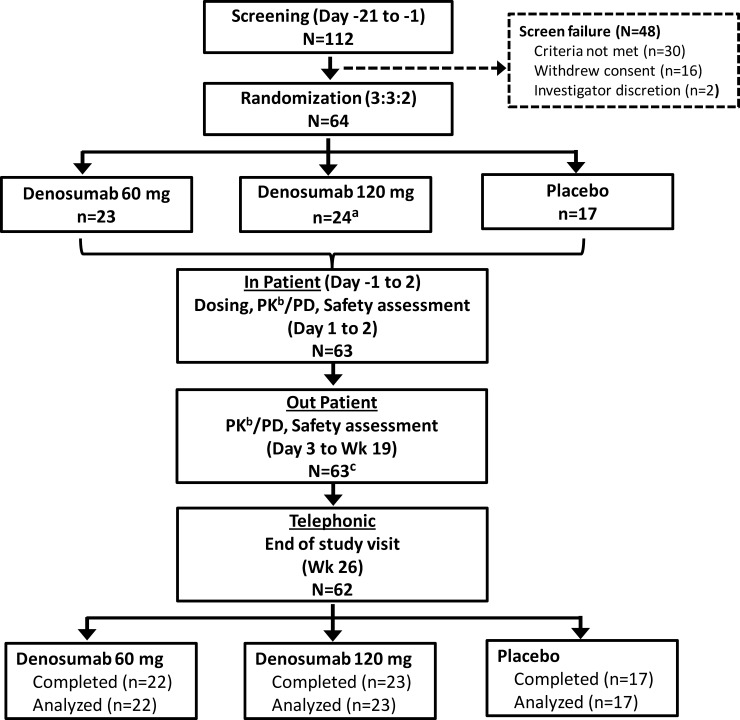
Fig 1. Study design and volunteers disposition.
aOne volunteer withdrew consent before administration of study treatment. bVolunteers from placebo group were not included for PK assessment. cOne volunteer who received denosumab 60 mg withdrew from study (investigator discretion; study day 147). PK, pharmacokinetic; PD, pharmacodynamic; Wk, week.