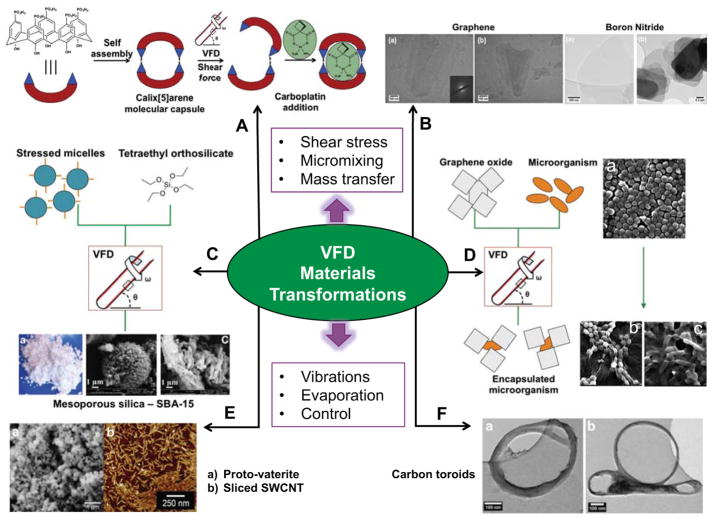
Figure 3.
VFD-mediated materials transformations. A) p-Phosphonate-calix[5]arene molecular capsules undergo H-bonding disruption (disassembly) under the shear stress for loading carboplatin post-VFD processing.[22] B) TEM images of exfoliated graphite and hexagonal boron nitride.[11d] C) Continuous flow processing of SBA-15 mesoporous silica where shear stressed micelles are mixed with triethyl orthosilicate (TEOS) and the resulting material filtered and calcinated, as with SEM images B and C.[26–27] D) VFD-mediated encapsulation of staphylocococus aureus and rhodococcus opacus graphene oxide sheets, SEM A - Staphylocococus aureus before processing in the VFD, SEM B - Staphylocococus aureus encapsulated in graphene oxide and SEM C - Rhodococcus opacus encapsulated in graphene oxide.[29] E) Controlling the rotational speed of the sample tube allows access to metastable forms of calcium carbonate such as proto-vaterite (SEM A), and sliced SWCNT (SEM B).[9b, 24] F) Toroids of self assembled SWCNT in a 1:1 water: toluene solvent mixture, where the high shear stress bends the SWCNT around the hydrophobic-hydrophilic interface.[11c]