Figure 4.
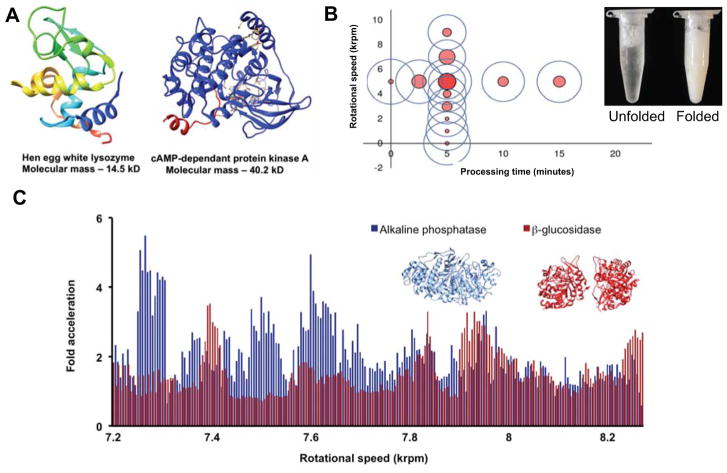
An overview of VFD-mediated biochemical transformations. A) Hen egg white lysozyme (HEWL) as a smaller protein with a molecular mass of 14.5 kDa and cAMP-dependant protein kinase A (PKA) as a larger protein with a molecular mass of 40.2 kDa. Both proteins were folded via VFD-mediated processing with a 100-fold decrease in refolding time.[7a] B) The dependency on the rotational speed of the sample tube on the capability of the VFD to refold HEWL, with rotational speed of 5.00 krpm for a 10 mm external diameter tube being effective. C) The acceleration landscapes for alkaline phosphatase and β-glucosidase compared to the non-VFD control. The specific rotational speeds needed to accelerate each enzyme hints towards a tertiary structure dependency on the correct rotational speed. For error calculations and statistical analysis of the data, see the original publication.[9a] D) An array of 28 stripes of mCherry and GFP protein on the inner surface of the VFD sample tube. Stripes are created through rapid bioconjugation of proteins fused to Hisn-tags through immobilized metal affinity chromatography (IMAC) resin bound to the inner surface of the sample tube.[29]