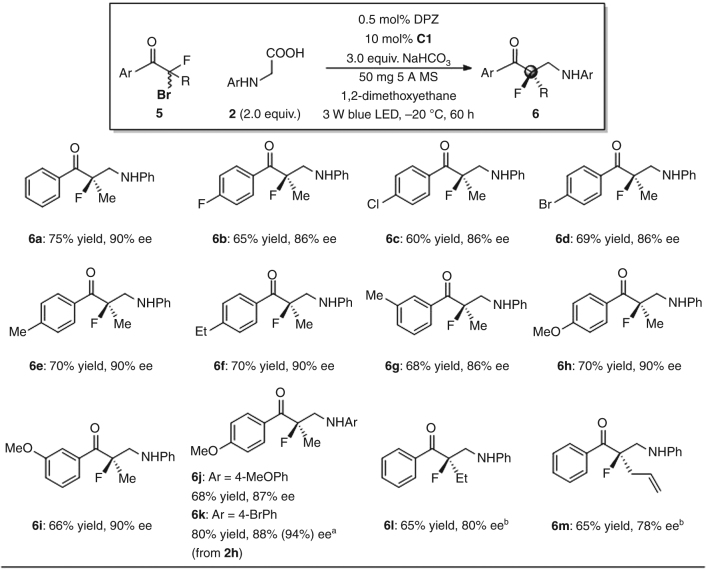
Fig. 3.
Substrate scope of tertiary α-bromo-α-fluoroketones with N-aryl glycines. Reactions were performed with 5 (0.1 mmol), 2 (0.2 mmol), DPZ (0.5 × 10−3 mmol), C1 (0.01 mmol), NaHCO3 (0.3 mmol), and 5 Å MS (50 mg) in 1,2-dimethoxyethane (1.5 mL) at −20 °C. Yields were determined from the isolated material after chromatographic purification. Enantiomeric excesses were determined by HPLC analysis on a chiral stationary phase. aThe ee value in parenthesis was obtained after recrystallization. bCatalyst C3 (20 mol%) was used instead of C1 and T = −45 °C