Abstract
Background
The transvenous implantable cardioverter‐defibrillator (ICD) lead is the most common source of complications in a traditional ICD system. This investigation aims to determine the incidence, predictors, and costs associated with these complications using a large insurance database.
Methods and Results
Data from the OptumLabs™ Data Warehouse, which include diagnosis, physician and procedure codes, and claims from patient hospitalizations, were analyzed. Patients with a de novo ICD or cardiac resynchronization therapy defibrillator implanted from January 1, 2003, through June 30, 2015, were included; those who did not have continuous coverage beginning 1 year before implantation were excluded, resulting in 40 837 patients followed up over an average of 2.3±2.1 years. Patients were followed up until they had the procedure or their last active date in the database. Of 20 580 device procedures, 2165 (5.3%) and 771 (1.9%) had mechanical and infectious complications, respectively. The 5‐year rate of freedom from mechanical complication was 92.0% and 89.3% for ICDs and cardiac resynchronization therapy defibrillators, respectively. Infectious complications were more likely in patients with a history of atrial fibrillation, diabetes mellitus, and renal disease, and the risk increased with subsequent device procedures. Younger age, female sex, lack of comorbidities, and implantations between 2003 and 2008 were associated with more mechanical complications.
Conclusions
Incidence of mechanical and infectious complications of transvenous ICD leads over long‐term follow‐up is much higher in the real world than in clinical studies. In our study cohort, 1 of 4 transvenous ICD leads had mechanical complications when followed up to 10 years. The high rate of reintervention leads to additional complications.
Keywords: complication, implantable cardioverter‐defibrillator, infection
Subject Categories: Arrhythmias, Catheter Ablation and Implantable Cardioverter-Defibrillator, Sudden Cardiac Death, Ventricular Fibrillation, Electrophysiology
Clinical Perspective
What Is New?
A large claims database found a high rate of lead mechanical and infectious complications over extended follow‐up, with the risk for mechanical complications being higher in younger, female, nonischemic patients implanted with cardiac resynchronization therapy defibrillator or with transvenous leads before 2008; infection risk is higher in male sex, patients with diabetes mellitus, atrial fibrillation, and chronic kidney disease and increases dramatically with each additional procedure.
What Are the Clinical Implications?
Strategies should be implemented to reduce the risk of transvenous implantable cardioverter‐defibrillator lead related complications, particularly in patients identified with increased risk or extended life expectancy, such as careful consideration given to the choice of leads or nontransvenous implantable cardioverter‐defibrillator systems and reduction of the number of repeated procedures by using devices with longer battery life.
Introduction
Implantable cardioverter‐defibrillators (ICDs) have been proven to reduce mortality in patients at risk for developing ventricular arrhythmias and in patients with a history of sustained ventricular tachycardia or cardiac arrest.1, 2, 3 Approximately 150 000 patients each year have a transvenous defibrillator implanted, of which ≈75% are for a primary prevention of sudden cardiac death.4 A percentage of transvenous system malfunctions is attributable to lead‐related problems and may require additional intervention.5 The transvenous ICD lead has been called the “weakest” link in ICD therapy and the most frequent source of serious complications associated with ICDs.6, 7, 8
The purpose of this investigation is to determine the incidence of ICD lead related complications over time, clinical factors associated with complications, and the costs associated with these complications in a large cohort of patients treated in general clinical practice.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Data Source
This study analyzed data from the OptumLabs Data Warehouse, an administrative claims database that includes deidentified data for privately insured and Medicare Advantage enrollees in a large, private, US health plan of >100 million enrollees over the past 20 years.9 The database contains longitudinal health information about enrollees, representing a diverse mixture of ages, ethnicities, and geographical regions across the United States. The health plan provides comprehensive insurance coverage for medical and prescription drug services. The data include diagnosis and procedure codes conforming to International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) classification, Current Procedural Terminology, Version 4 procedure codes, and Healthcare Common Procedure Coding System procedure codes; the database uses claims from inpatient and outpatient hospital codes as well as physician codes. The data are deidentified and compliant with the Health Insurance Portability and Accountability Act of 1996. Our study was exempt from institutional review board approval because we only used preexisting deidentified data.
Study Population
Patients with a de novo ICD or cardiac resynchronization therapy defibrillator (CRT‐D) device implanted from January 1, 2003, through June 30, 2015, were included. De novo ICD and CRT‐D patient procedures were identified if a first claim of ICD implantation (inpatient code 37.94 or 37.96; outpatient code 33240 or 33249, without 33225) or CRT‐D implantation (inpatient code 00.51; outpatient code 33249 and with 33225 within 7 days) occurred. Patients with a first implantation procedure that also had a device explant code were excluded, to account for cases of a previous device implantation under a different coverage database (Table 1 and Figure S1). Patients with continuous coverage spanning the de novo implantation procedure were included to determine study period start and end dates.
Table 1.
Procedure and Diagnosis Codes
| Code | Description | Category (First Device Procedure) | Category (Intervention During Follow‐Up) |
|---|---|---|---|
| Procedure codes | |||
| 33215 | Reposition pacing defibrillator lead | Exclusion criteria | Revise/remove lead |
| 33216 | Revision implanted electrode | Exclusion criteria | Revise/remove lead |
| 33217 | Insert/revise electrode | Exclusion criteria | Revise/remove lead |
| 33218 | Repair lead pacemaker‐defibrillator, one | Exclusion criteria | Revise/remove lead |
| 33220 | Repair lead pacemaker‐defibrillator, dual | Exclusion criteria | Revise/remove lead |
| 33244 | Remove electrode, transvenous | Exclusion criteria | Revise/remove lead |
| 37.76 | Replace transvenous lead | Exclusion criteria | Revise/remove lead |
| 37.97 | Replace cardioverter/defibrillator lead | Exclusion criteria | Revise/remove lead |
| 37.75 | Revision pacemaker lead | Exclusion criteria | Revise/remove pacing lead |
| 37.77 | Removal of pacing lead without replacement | Exclusion criteria | Revise/remove pacing lead |
| 33235 | Removal pacemaker electrode | Exclusion criteria | Revise/remove pacing lead |
| 33226 | Reposition LV lead | Exclusion criteria | LV lead |
| 33225 | Insert pacing electrode (LV) | CRT‐D if 33249 within 7 d | LV lead |
| 0.52 | Implant/replace transvenous lead into LV coronary venous system | LV lead | |
| 33249 | Insert/replace defibrillator system with lead(s) |
ICD if coded alone CRT‐D if 33225 within 7 d |
Device and leads |
| 37.94 | Insert/replace defibrillator, total system | ICD | Device and leads |
| 0.51 | Implant CRT‐D total system | CRT‐D | Device and leads |
| 33240 | Insert PG | ICD | Remove/replace PG |
| 33241 | Remove PG | Exclusion criteria | Remove/replace PG |
| 33262 | (2012+) Removal of ICD with replacement; single‐lead system | Exclusion criteria | Remove/replace PG |
| 33263 | (2012+) Removal of ICD with replacement; dual‐lead system | Exclusion criteria | Remove/replace PG |
| 33264 | (2012+) Removal of ICD with replacement; multilead system | Exclusion criteria | Remove/replace PG |
| 37.87 | Replace any device with dual chamber | Exclusion criteria | Remove/replace PG |
| 37.89 | Revise/remove pacemaker | Exclusion criteria | Remove/replace PG |
| 37.96 | Insert cardioverter/defibrillator PG | ICD | Remove/replace PG |
| 37.98 | Replace cardioverter/defibrillator PG | Exclusion criteria | Remove/replace PG |
| 0.54 | Implant/replace CRT‐D generator only | Exclusion criteria | Remove/replace PG |
| 37.79 | Revise pacemaker pocket | Exclusion criteria | Revise pocket |
| 33223 | Pacemaker AICD pocket | Exclusion criteria | Revise pocket |
| Complication Type | Diagnosis Code | Description |
|---|---|---|
| Diagnosis codes | ||
| Mechanical | 996 | Mechanical complication of cardiac device implantation and graft |
| 996 | Mechanical complication of unspecified cardiac device, implant, and graft | |
| 996.01 | Mechanical complication attributable to cardiac pacemaker (electrode) | |
| 996.04 | Mechanical complication of automatic implantable cardiac defibrillator | |
| 996.09 | Other mechanical complication of cardiac device, implant, and graft | |
| 996.7 | Other complications because of unspecified device, implant, and graft | |
| 996.72 | Other complications because of other cardiac device, implant, and graft | |
| Infection | 996.61, 996.62, 996.6, 996.60 | Infection attributable to device, implant, and graft |
| 038.(0, 10, 11, 19, 2, 3, 40, 41, 42, 43, 44, 49, 8, 9) | Sepsis | |
| 790.7 | Bacteremia | |
| 421, 421.(0, 1, 9) 424.(9, 90, 91, 99) | Endocarditis | |
| 785.5 | Shock | |
| 682.8, 682.9 | Cellulitis | |
AICD indicates automatic implantable cardiac defibrillator; CRT‐D, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter‐defibrillator; LV, left ventricular; PG, pulse generator.
Outcomes
The time between a de novo ICD implantation and the first lead procedure that also included a diagnosis code indicating mechanical complication or infection was calculated. Diagnosis codes and lead reintervention categories are shown in Table 1. Patients were followed up until they had a lead revision or until their last active date in the database. Defibrillator lead revisions were captured using inpatient ICD‐9 codes 37.76 or 37.97 or outpatient codes 33215, 33216, 33217, 33218, 33220, or 33244.
Demographics
Patients were required to have a minimum of 12 months of coverage in the database before the de novo implantation to capture baseline comorbidities. Disease entities from the Charlson Comorbidity Index were used (myocardial infarction, congestive heart failure, peripheral vascular disease, chronic pulmonary disease, diabetes mellitus without chronic complications, diabetes mellitus with chronic complications, renal disease, and moderate or severe liver disease).10 Codes used to extract comorbidities are shown in Table S1.
Cost of Procedure
The database uses a standardized pricing model to create a uniform and consistent approach to classifying and pricing all services, from the payer perspective. This process is designed to remove variations that may be driven by differences in contractual arrangements, geographic regions, time frames of data, and the healthcare setting or organization from which services are provided.
The process is designed to convert relative values to baseline dollar unit prices, using conversion factors that reflect typical managed care payment levels. This process, in turn, creates the baseline or standard cost for each type of service using relative value units and the conversion factor.
For this analysis, the cost for each patient was summed over the 15 days before and after de novo procedure and incident complication, with additional procedures to create a 30‐day cost surrounding the procedure. The costs for each 30‐day period relative to the de novo or additional procedure(s) attributable to complication were also calculated for each patient.
Statistical Analyses
Descriptive statistics are reported using frequency and percentage for categorical variables and mean and SD for continuous variables. Kaplan‐Meier analyses were used to estimate the time to first event for complications. Patients were censored at their coverage end date or June 30, 2015. Analysis was performed in 2016, allowing for complete reporting of events during the study period. Time‐to‐event analysis was performed from implantation through all available follow‐up and separately for the peri‐implantation period for the first 91 days and the long‐term implantation period after 92 days. These time periods were chosen to align with the current US regulatory reporting criteria for lead surveillance studies. Patients with a complication in the first 91 days were censored in the analysis of the time‐to‐first complication in the long‐term period starting at day 92, so that acute and chronic complication rates do not get counted more than once.
A univariate Cox regression model was used to assess time to first lead reintervention by patient demographic and comorbidities. Each risk factor with P<0.10 in univariate analysis was subsequently analyzed in a multivariate model. Backward selection was used, and all covariates with P<0.05 were retained in the multivariate model.
The risk of complication was also assessed after each additional non–end point device procedure during follow‐up. To prevent inappropriate inclusion of implantation‐ or complication‐related procedures in this analysis, a 7‐day blanking period was used after de novo implantation and before a complication procedure. The presence or absence of additional complication risk was assessed in a time‐varying covariate model, including the number of each additional non–end point procedure (1, 2, 3, or ≥4 procedures), time of the procedure, and all baseline covariates. Therefore, for example, patients who had 3 procedures without associated diagnosis codes for a mechanical complication or infection would have their risk of an end point assessed in 4 different periods (after the de novo procedure as well as after each additional procedure). All analyses were performed with SAS, version 9.4, and R, version 3.2.
Results
Study Cohort and Patient Characteristics
Of 107 547 patients with an ICD or CRT‐D implantation in the database, 84 268 had a de novo implantable defibrillator procedure meeting inclusion criteria. Excluding patients without continuous enrollment spanning the de novo procedure and patients without 1 year of continuous coverage before the de novo procedure, 40 837 patients were included in the analysis.
Patients were primarily men (73%), and with an average age of 61.7±12.8 years. Patient characteristics are shown in Table 2. Patients were followed up for an average of 2.3±2.2 years in this analysis (range, 0.1–12.1 years). The numerical and temporal distribution of patient follow‐up is shown in Figure S2. The risk of each event was evaluated using standard Kaplan‐Meier analysis, which includes the number patients who were followed up for each point in time as being “at risk,” although the average follow‐up for the full cohort may be shorter than any given Kaplan‐Meier estimate. In other words, the 5‐year data are based on the 4540 patients who had prolonged follow‐up.
Table 2.
Baseline Demographics and Comorbidities
| Variable | CRT‐D Group | ICD Group | Overall |
|---|---|---|---|
| No. | 12 300 | 28 537 | 40 837 |
| Age, mean±SD, y | 65.5±11.5 | 60.0±13.0 | 61.7±12.8 |
| Male sex, % | 69.8 | 74.4 | 73.0 |
| Comorbidities, % | |||
| Myocardial infarction | 30.9 | 41.8 | 38.6 |
| Atrial fibrillation | 41.7 | 32.0 | 34.9 |
| Heart failure | 95.4 | 72.9 | 79.7 |
| Peripheral vascular disease | 18.1 | 16.5 | 17.0 |
| Hypertrophic cardiomyopathy | 2.4 | 5.2 | 4.3 |
| Chronic pulmonary disease | 30.8 | 25.1 | 26.8 |
| Diabetes mellitus (uncomplicated) | 44.0 | 36.8 | 39.0 |
| Diabetes mellitus (complicated) | 17.6 | 14.0 | 15.1 |
| Renal disease | 21.9 | 15.1 | 17.1 |
| Liver disease | 3.7 | 3.4 | 3.5 |
CRT‐D indicates cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter‐defibrillator.
End Point Event Rates
Of 20 580 device procedure codes that were included in the analysis, 2165 (5.3% of study cohort) had an associated mechanical complication from the ICD lead, whereas 771 (1.9% of study cohort) had an infectious complication involving the ICD lead over the average follow‐up of 2.3 years.
The 5‐year rate of freedom from mechanical complication was 92.0% for ICD and 89.3% for CRT‐D. In patients who had longer follow‐up, the 10‐year freedom from mechanical complication was only 75% (Figure 1). Complications in the periprocedural phase (0–90 days after implantation) occurred in 2.2% of patients with an ICD and 2.9% of patients with a CRT‐D. Chronic complications increased most rapidly for patients with an ICD from year 6 to 8 and from year 8 to 10, incrementing by 7% for each biannual increment in follow‐up from year 6 onwards. CRT‐D complications increased 5.5% from year 4 to 6.
Figure 1.
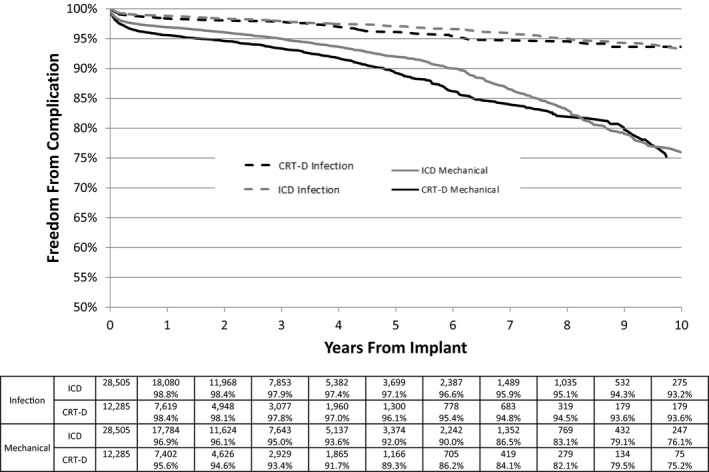
Kaplan‐Meier plot of lead revision associated with a diagnosis of infection (dashed line) or mechanical complications (solid line) for patients with an implantable cardioverter‐defibrillator (ICD; gray line) or cardiac resynchronization therapy defibrillator (CRT‐D; black line).
The 5‐year rate of freedom from an infectious complication necessitating a procedure was 97.1% and 96.1% for patients with an ICD and a CRT‐D, respectively. Acute infections (occurring in the first 90 days after implantation) occurred in 0.9% of patients.
Baseline Factors Associated With Complication Events
Mechanical complications
Univariate analysis for the effect of patient demographics on lead mechanical complications or infection was analyzed individually for the peri‐implantation (0–90 days) period and the long‐term (>90 days) period. Female sex, CRT‐D device, and absence of comorbidities, including complicated diabetes mellitus and previous myocardial infarction, were associated with peri‐implantation mechanical complications. Long‐term mechanical complications were associated with younger age at implantation (age <65 years), female sex, CRT‐D device, and absence of comorbidities, including history of myocardial infarction and peripheral vascular disease. Implantations from 2003 to 2008 had the greatest risk of long‐term mechanical complications (Figure 2). These univariate factors remained significant in the multivariate model, with the exception of complicated diabetes mellitus (peri‐implantation) and peripheral vascular disease (long‐term) (Figure S3).
Figure 2.
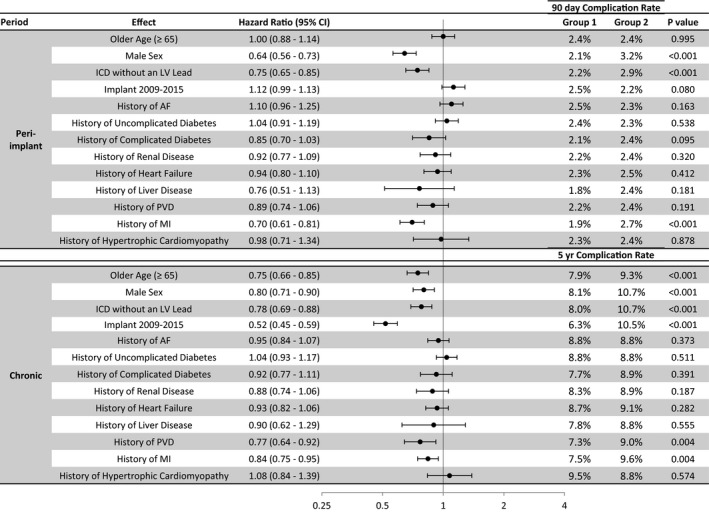
Univariate analysis of risk factors for lead procedures with mechanical diagnosis. Baseline variables associated with an increased risk of mechanical complication during the peri‐implantation and long‐term period. The 90‐day and 5‐year Kaplan‐Meier estimates are shown for each group. AF indicates atrial fibrillation; CI, confidence interval; ICD, implantable cardioverter‐defibrillator; LV, left ventricular; MI, myocardial infarction; PVD, peripheral vascular disease.
Infection complications
Atrial fibrillation, diabetes mellitus, renal disease, and peripheral vascular disease were associated with increased risk of peri‐implantation infectious complications and CRT‐D device. Patients with age <65 years at implantation, male sex, diabetes mellitus, renal disease, and heart failure had a higher risk of chronic infectious complications (Figure 3). Only diabetes mellitus and renal disease were associated with both peri‐implantation and chronic infection complications, along with a history of atrial fibrillation for peri‐implantation and male sex for chronic complications (Figure S3).
Figure 3.
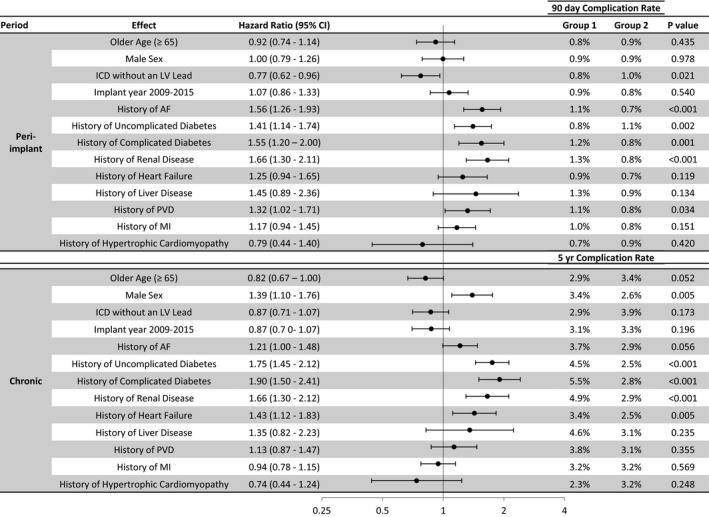
Univariate analysis of risk factors for lead procedures with infectious diagnosis. Baseline variables associated with an increased risk of infectious complication during the peri‐implantation and long‐term period. The 90‐day and 5‐year Kaplan‐Meier estimates are shown for each group. AF indicates atrial fibrillation; CI, confidence interval; ICD, implantable cardioverter‐defibrillator; LV, left ventricular; MI, myocardial infarction; PVD, peripheral vascular disease.
Procedures During Follow‐Up and Association With Complication Events
In a time‐varying model including device procedures during follow‐up, each of the baseline variables remained significant, but the risk was decreased slightly for each variable, whereas each additional procedure almost doubled the risk of an infectious complication (Figure 4 top). Additional procedures were not significantly associated with mechanical complications, although a trend toward increased risk was seen for ≥2 procedures (Figure 4 bottom).
Figure 4.
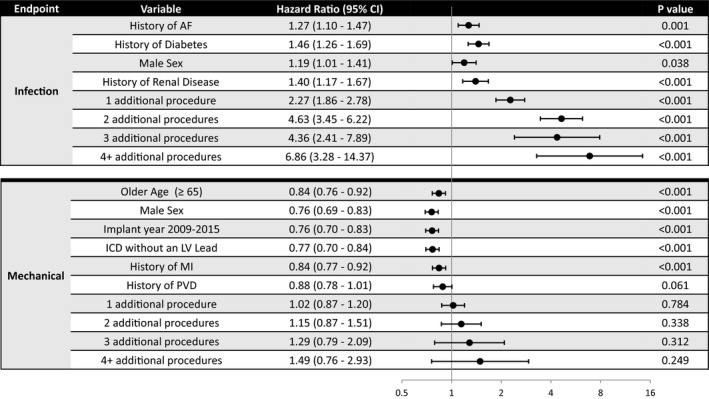
Time‐varying covariate model for the risk of infectious or mechanical implantable cardioverter‐defibrillator (ICD) lead complication(s). Risk of complications per baseline variables and noncomplicated procedures in time‐varying multivariable model. AF indicates atrial fibrillation; CI, confidence interval; LV, left ventricular; MI, myocardial infarction; PVD, peripheral vascular disease.
Cost of Complications Associated With ICD and CRT‐D Procedures
The average costs surrounding the de novo implantation procedure was slightly higher in patients who went on to have system revisions because of infections ($74 207 versus $65 757 for patients without future complications), as were the total healthcare quarterly costs after the implantation procedure. In contrast, patients who went on to have lead revisions because of mechanical complications had similar de novo implantation procedure costs ($64 154) and quarterly costs as patients who did not have any complications (Figure 5 top). The cost of the revision procedure for system revisions because of infectious complications ($68 211) was higher than for revisions because of mechanical complications ($36 283), as were the quarterly costs before and after revision (Figure 5 bottom).
Figure 5.
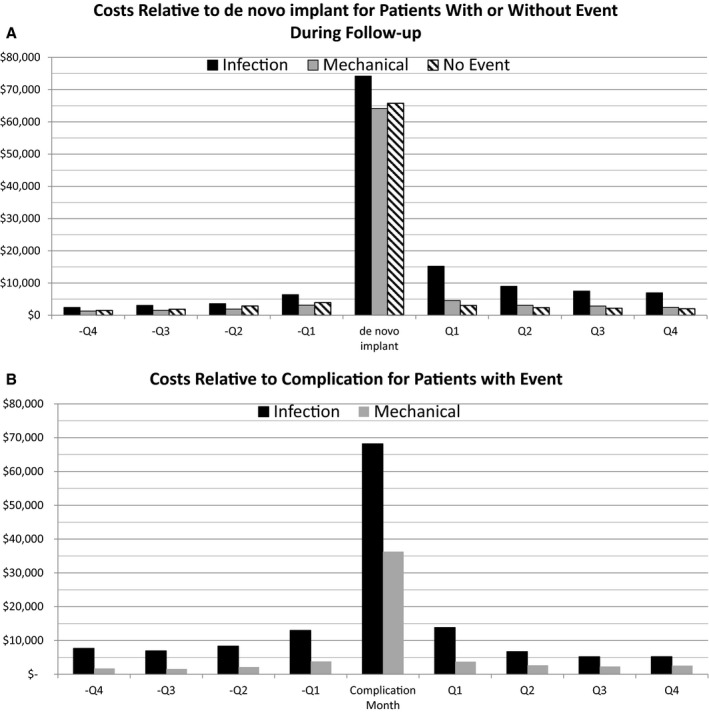
Costs relative to de novo and complication procedure. The costs during the de novo procedure month (top) and the complication procedure month (bottom) grouped by type of complication the patient experienced during follow‐up. Months before and after procedure are shown in quarters (Qs).
During the de novo procedure, 59.9% of patients had an inpatient hospitalization, with average length of stay of 6.7 days. The procedure to address mechanical complications had a lower hospitalization rate (51.5%) and similar length of stay (5.2 days) as the de novo procedure. Infectious complications resulted in higher hospitalization rates (82.4%) and increased hospital stays (16.5 days).
Discussion
The ability to glean data from large healthcare databases has allowed researchers and clinicians to analyze the incidence of ICD lead failure, the Achilles heel of a transvenous ICD. Lead failure attributable to mechanical causes usually results in a snowballing effect, with incremental numbers of procedures performed to rectify the problem. Such interventions have a high incidence of unsavory outcomes, which in certain instances could result in patient death during a lead extraction and infection with virulent organisms, leading to significant financial burden imposed on the patient and the healthcare systems. Prior studies that evaluated ICD lead reliability were based on single‐center or multicenter studies and industry‐related databases. These studies are limited by insufficient patient follow‐up time and/or small sample size. The present analysis is unique in that it includes a large number of patients with long‐term follow‐up and implanted with ICD leads from different manufacturers and by implanters in varied healthcare settings, both private and academic.
The main findings from our study are as follows:
There are a significant number of mechanical complications that occur with transvenous ICD leads, with up to 1 in 4 patients experiencing a complication over 10 years of follow‐up. In the total sample, female patients, age <65 years, implantation of CRT‐D compared with ICD, and nonischemic cause for heart failure were associated with more mechanical complications, as were leads implanted before 2008.
Comorbidities play a major role in infectious complications of ICD and CRT‐D implants; diabetes mellitus, atrial fibrillation, male sex, and chronic kidney disease were more frequently associated with infectious complications.
Incremental numbers of procedures significantly increased the risk of infection and result in prolonged hospitalizations and significantly higher healthcare costs, compared with mechanical complications.
In our study, absence of comorbidities and younger age were associated with greater risk of long‐term mechanical complications, likely because younger, healthier patients are expected to live longer and have longer lead dwell times, thereby increasing the risk for ICD lead malfunction. In addition, younger patients mount a more robust immunological response to ICD leads and likely exert greater mechanical forces that can disrupt the structural integrity of the leads compared with more elderly cohorts. Patients with diabetes mellitus and chronic kidney disease may be more likely to develop calcium deposits on ICD leads, which can increase their risk of lead failure. In addition, these 2 conditions, as well as presence of comorbidities in general, also portend a higher risk of infection. It is being increasingly recognized that the risks and benefits of transvenous ICDs may differ in patients with heart failure and chronic kidney disease. In a recent study, all‐cause mortality did not differ in eligible patients with chronic kidney disease who received an ICD versus those who did not. The authors speculated that increasing hospitalizations because of worsening heart failure and infectious complications could have nullified the benefits of primary prevention.11
The risk of mechanical failure of transvenous ICD leads, 25% at 10 years, was higher than may be expected. However these data are consistent with other studies when long‐term follow‐up and recalled leads are included.12 The data in our analysis closely resemble data from Borleffs et al, in whose study the lead failure at 1 year was 1.9%; at 2 years, 3.5%; at 5 years, 10.4%; and at 10 years, 26.9%.13
The time span of follow‐up (2003–2015) includes leads subject to recall because of significantly increased mechanical failure rates (Sprint Fidelis, US commercial release in September 2004 and recall in October 2007; Riata and Riata ST, US commercial release in March 2002, supplanted by Durata in September 2007, and recall in December 2010). The product performance reports list failure rates similar to those found in this analysis; Sprint Fidelis 10‐year survival is 79%, and Riata/Riata ST 10‐year survival is 87%.14, 15 The claims database used in the current analysis does not include the make and model of defibrillation lead, preventing a separate analysis of recalled versus nonrecalled transvenous leads. However, an analysis of the failure rates on the basis of implantation year found the leads implanted in 2005, 2006, and, to a lesser extent, 2007 had increased failure rates compared with both leads implanted before 2005 and after 2007 (Figure 6). Therefore, although some leads may exhibit higher lead reliability than was found in this analysis, taken as a whole, the population of transvenous defibrillation leads since 2003 has exhibited high mechanical complication rates over 10 years of follow‐up.
Figure 6.
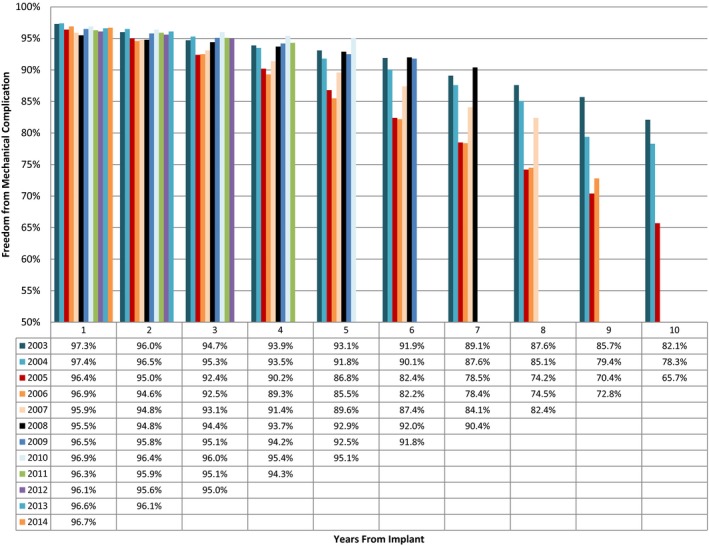
Mechanical complication by year of implantation. The freedom from mechanical complication, as calculated by Kaplan‐Meier analysis, for leads implanted in each year between 2003 and 2014 over a 10‐year follow‐up. Noticeably, leads implanted in 2005, 2006, and 2007 have increased failure rates compared with leads implanted before or after that time period.
The increased incidence of lead failure in women in this analysis may be related to anatomic issues related to lead stress or body mass, and it is consistent with prior studies showing a higher rate of lead perforation or complications from lead extraction.14, 15, 16 Lead failure portends a particular problem in patients with comorbidities that need extraction. In the present study, diabetes mellitus and renal disease were independent predictors of short‐ and longer‐term infectious complications. Atrial fibrillation was associated with short‐term infectious complications, and male sex was associated with long‐term infectious complications. This is consistent with a review of the literature that also found prior cardiovascular implantable electronic device infection, cardiovascular implantable electronic device intervention, and cardiovascular implantable electronic device replacement to be strong predictors of infection risk.16
Maytin et al17 showed that the instantaneous mortality rate increases by 5% per each year of patient age and by 16% for each mg/dL increase in serum creatinine; presence of diabetes mellitus increases the rate of death by 71%, and the indication for lead extraction has the most significant impact on survival. Patients with systemic and local infections have a 3.5‐ and a 2.7‐fold increased rate of death, respectively.17
Thus, the choice of implantation of ICDs, despite their proven benefit, should always include the patient profile and comorbidities. Informed choices need to be made about the types of defibrillators and the types of ICD leads. This is not one size fits all.18
The risks of mechanical complications and infections described in this analysis must be weighed against the prophylactic protection against sudden cardiac arrest in at‐risk patients. Although the subcutaneous ICD system has not had a similar large, multiple‐institution, multiyear analysis, as performed herein, the subcutaneous defibrillation electrode has had a mechanical complication rate of 6/45 000 implants worldwide19 and may provide a different risk profile. Potential explanations include absence of the beat‐to‐beat mechanical stress that the transvenous ICD leads endure and fundamentally different lead design in an extravascular space. The equivalence of efficacy of treatment of the subcutaneous ICD and transvenous ICD systems has been well validated.20, 21
Our study is unique in that temporal trends of transvenous ICD lead failure were evaluated for ICD and CRT‐D de novo implants and the financial burden associated with lead failure. The incremental financial burden associated with reintervention in our study was $68 000 to treat an infection and $36 000 to treat a mechanical failure, and the average costs for the year before and the year after a complication were elevated if a patient had an infection. This is in consonance with other studies that suggest that the healthcare expenditure associated with infections and reinterventions could be astronomical.22, 23, 24
The strong correlation between prior lead procedures and infection demands careful scrutiny in determining methods to minimize invasive procedures. Recent data from the ICD Registry found 40% of ICD procedures were revision procedures for battery depletion or upgrade4; the reason for increasing infection rates, as found by Greenspon et al, may be because of a higher proportion of device replacement procedures, including generator replacement for premature battery depletion.22 Our data extend their observations and further emphasize the morbidity and financial impact of their findings.
Study Limitations
This study has several important limitations. We included data from a claims database that is derived largely from private payer systems. The population age is similar to transvenous ICD studies and is younger than other analysis of claims data based on a Medicare population. Our results would not necessarily be exactly applicable to older and indigent patient populations.
Our study is based on administrative claims data and, thus, does not include some data typically collected in a clinical study, such as indication for the device, ejection fraction, QRS width, and other clinical measures that may be important risk factors. Some clinical events may not be identifiable through claims data or lack specificity, such as arrhythmic events and type of arrhythmia. Study end points were identified through diagnosis codes and rely on correct reporting. Severity of complication could not be assessed, and differing types of infectious and mechanical complications were not distinguished.
The database did not include patient mortality; therefore, patient survival could not be evaluated. Patient death reporting from the Social Security Death Index was altered in 2011 because of a change in the Social Security Act, resulting in approximately a third of patient deaths not being reported; thereby, this caused decreased confidence in mortality analyses.
Device manufacturer and model were not available and, therefore, the analysis could not identify patients with recalled leads.
Costs are based on standardized prices and may not reflect the true cost of providing services at any one institution.
Conclusions
Lead‐related complications remain a significant cause of morbidity and mortality associated with the transvenous ICD system, in addition to incurring an unacceptably high financial burden for patients, caregivers, and the healthcare system. Reliability of ICD leads at 5 and 10 years continues to be disappointing. Careful consideration should be given to the choice of leads or nontransvenous ICD systems, particularly for patients at a higher risk for mechanical complications or infection.
Sources of Funding
This work was sponsored by Boston Scientific.
Disclosures
Koneru reports honoraria from Boston Scientific, St Jude Medical, and Medtronic. Ellenbogen reports consulting, honorarium, and research grants from Boston Scientific and Medtronic; and consulting and honorarium from St Jude Medical (Abbott) and Biotronik (honorarium). Jones, Hammill, and Wold are employees of Boston Scientific Corp.
Supporting information
Table S1. Demographic Codes
Figure S1. Analysis method for capturing de novo, uncomplicated, and complicated procedures. 7‐day blanking period to prevent inappropriate inclusion of implant or complication procedures (ie, same procedure incorrectly coded on different dates for inpatient/outpatient/physician).
Figure S2. Patient follow‐up in study.
Figure S3. Multivariate analysis of risk factors for complications.
(J Am Heart Assoc. 2018;7:e007691 DOI: 10.1161/JAHA.117.007691.)29748177
References
- 1. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 2. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML; Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 3. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter—defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 4. Kremers MS, Hammill SC, Berul CI, Koutras C, Curtis JS, Wang Y, Beachy J, Blum Meisnere L, Conyers DM, Reynolds MR, Heidenreich PA, Al‐Khatib SM, Pina IL, Blake K, Norine Walsh M, Wilkoff BL, Shalaby A, Masoudi FA, Rumsfeld J. The National ICD Registry Report: version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013;10:e59–e65. [DOI] [PubMed] [Google Scholar]
- 5. Friedman DJ, Parzynski CS, Varosy PD, Prutkin JM, Patton KK, Mithani A, Russo AM, Curtis JP, Al‐Khatib SM. Trends and in‐hospital outcomes associated with adoption of the subcutaneous implantable cardioverter defibrillator in the United States. JAMA Cardiol. 2016;1:900–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maisel WH. Transvenous implantable cardioverter‐defibrillator leads: the weakest link. Circulation. 2007;115:2461–2463. [DOI] [PubMed] [Google Scholar]
- 7. Kleemann T, Becker T, Doenges K, Vater M, Senges J, Schneider S, Saggau W, Weisse U, Seidl K. Annual rate of transvenous defibrillation lead defects in implantable cardioverter‐defibrillators over a period of >10 years. Circulation. 2007;115:2474–2480. [DOI] [PubMed] [Google Scholar]
- 8. Ezzat Va, Lee V, Ahsan S, Chow AW, Segal O, Rowland E, Lowe MD, Lambiase PD. A systematic review of ICD complications in randomised controlled trials versus registries: is our “real‐world” data an underestimation? Open Heart. 2015;2:e000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum labs: building a novel node in the learning health care system. Health Aff. 2014;33:1187–1194. [DOI] [PubMed] [Google Scholar]
- 10. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J‐C, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 11. Bansal N, Szpiro A, Reynolds K, Smith DH, Magid DJ, Gurwitz JH, Masoudi F, Greenlee RT, Tabada GH, Sung SH, Dighe A, Go AS. Long‐term outcomes associated with implantable cardioverter defibrillator in adults with chronic kidney disease. JAMA Intern Med. 2018;178:390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Providência R, Kramer DB, Pimenta D, Babu GG, Hatfield LA, Ioannou A, Novak J, Hauser RG, Lambiase PD. Transvenous implantable cardioverter‐defibrillator (ICD) lead performance: a meta‐analysis of observational studies. J Am Heart Assoc. 2015;4:e002418 DOI: 10.1161/JAHA.115.002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borleffs CJ, van Erven L, van Bommel RJ, van der Velde ET, van der Wall EE, Bax JJ, Rosendaal FR, Schalij MJ. Risk of failure of transvenous implantable cardioverter‐defibrillator leads. Circ Arrhythm Electrophysiol. 2009;2:411–416. [DOI] [PubMed] [Google Scholar]
- 14. Medtronic . Product performance report (2nd edition—issue 77). http://wwwp.Medtronic.Com. Accessed July 31, 2017.
- 15. Abbott . Product performance report, 2017 second edition. https://www.Sjm.Com. Accessed December 8, 2017.
- 16. Nielsen JC, Gerdes JC, Varma N. Infected cardiac‐implantable electronic devices: prevention, diagnosis, and treatment. Eur Heart J. 2015;36:2484–2490. [DOI] [PubMed] [Google Scholar]
- 17. Maytin M, Jones SO, Epstein LM. Long‐term mortality after transvenous lead extraction. Circ Arrhythm Electrophysiol. 2012;5:252–257. [DOI] [PubMed] [Google Scholar]
- 18. Al‐Khatib SM, Friedman P, Ellenbogen KA. Defibrillators: selecting the right device for the right patient. Circulation. 2016;134:1390–1404. [DOI] [PubMed] [Google Scholar]
- 19. BostonScientific . Product performance report (2018 q1 edition). http://www.Bostonscientific.Com. Accessed January 10, 2018.
- 20. Burke MC, Gold MR, Knight BP, Barr CS, Theuns DAMJ, Boersma LVA, Knops RE, Weiss R, Leon AR, Herre JM, Husby M, Stein KM, Lambiase PD. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2‐year results from a pooled analysis of the IDE study and effortless registry. J Am Coll Cardiol. 2015;65:1605–1615. [DOI] [PubMed] [Google Scholar]
- 21. Boersma LV, Barr CS, Burke MC, Leon AR, Theuns DA, Herre JM, Weiss R, Kremers MS, Neuzil P, Husby MP, Carter N, Stivland TM, Gold MR; EFFORTLESS and IDE Study Investigators . Performance of the subcutaneous implantable cardioverter‐defibrillator in patients with a primary prevention indication with and without a reduced ejection fraction versus patients with a secondary prevention indication. Heart Rhythm. 2016;14:367–375. [DOI] [PubMed] [Google Scholar]
- 22. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. 16‐Year trends in the infection burden for pacemakers and implantable cardioverter‐defibrillators in the United States: 1993 to 2008. J Am Coll Cardiol. 2011;58:1001–1006. [DOI] [PubMed] [Google Scholar]
- 23. Deshmukh A, Patel N, Noseworthy PA, Patel AA, Patel N, Arora S, Kapa S, Noheria A, Mulpuru S, Badheka A, Fischer A, Coffey JO, Cha YM, Friedman P, Asirvatham S, Viles‐Gonzalez JF. Trends in use and adverse outcomes associated with transvenous lead removal in the United States. Circulation. 2015;132:2363–2371. [DOI] [PubMed] [Google Scholar]
- 24. Reynolds MR, Cohen DJ, Kugelmass AD, Brown PP, Becker ER, Culler SD, Simon AW. The frequency and incremental cost of major complications among Medicare beneficiaries receiving implantable cardioverter‐defibrillators. J Am Coll Cardiol. 2006;47:2493–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographic Codes
Figure S1. Analysis method for capturing de novo, uncomplicated, and complicated procedures. 7‐day blanking period to prevent inappropriate inclusion of implant or complication procedures (ie, same procedure incorrectly coded on different dates for inpatient/outpatient/physician).
Figure S2. Patient follow‐up in study.
Figure S3. Multivariate analysis of risk factors for complications.


