Abstract
Background
Depression in patients with coronary artery disease (CAD) is associated with increased cardiovascular morbidity. Given the proinflammatory actions of phospholipids, aberrant phospholipid metabolism may be an etiological mechanism linking CAD and depression. Our primary objective was to identify a phospholipid biomarker panel that characterizes CAD patients with significant depressive symptoms from those without.
Methods and Results
We performed a targeted lipidomic analysis on CAD patients with significant depressive symptoms (n=37, Center for Epidemiologic Studies Depression score ≥16) and those without (n=49). Phospholipid species were selected using partial least‐square discriminant analysis, and the ability of the resulting model to discriminate between groups was evaluated using receiver operator characteristic curves. Biosignature scores were calculated from this model, and analyses of covariance were performed to compare intergroup differences in biosignature scores, with adjustment for clinical differences between patients. Those with significant depressive symptoms had lower cardiopulmonary fitness, more prevalent history of depression, and a greater number of vascular risk factors. A model of 10 phospholipid species had an area under the curve value of 0.84 (95% confidence interval 0.72‐0.95), sensitivity of 0.73, and specificity of 0.71. This model passed permutation testing (n=1000, P<0.001). Biosignature scores were higher in those with significant depressive symptoms after adjustment for potential confounders (F[1.86]=14.39, P<0.0005).
Conclusions
The present findings support the role of proinflammatory phospholipid species in the presence of depression in CAD patients from the CAROTID trial (Coronary Artery Disease Randomized Omega‐3 Trial in Depression). Future investigations should aim to replicate findings in larger data sets and clarify possible pathophysiological mechanisms.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00981383.
Keywords: cardiac rehabilitation, coronary artery disease, depression, inflammation, lipidomics, lipids
Subject Categories: Coronary Artery Disease, Mental Health, Biomarkers, Clinical Studies, Lipids and Cholesterol
Clinical Perspective
What Is New?
Although depression in coronary artery disease patients is associated with worse cardiac outcomes, little is known about the biochemical connection between coronary artery disease and depressive symptoms.
This study identifies a 10‐phospholipid biomarker panel that discriminates coronary artery disease patients with significant depressive symptoms from those without (area under the curve=0.84 [0.72, 0.95]).
Composite scores comprising phospholipids from this panel differed significantly between these 2 groups.
What Are the Clinical Implications?
Those with significant depressive symptoms had higher concentrations of saturated and ω‐6 phospholipids, suggesting that downstream inflammatory signals are a primary contributor to depression in coronary artery disease.
Given the clinical consequences of depression in those living with heart disease, future validation of this biomarker model could help identify patients at risk for poor prognosis and provide new directions for therapeutic interventions aimed at neuroinflammation.
Introduction
In the past few decades, exercise‐based cardiac rehabilitation has been instrumental in decreasing the cardiovascular mortality rate of patients with coronary artery disease (CAD).1, 2 However, CAD remains a leading cause of mortality, accounting for 1 in 7 deaths in the United States.3 A barrier to maximizing benefit from cardiac rehabilitation is the frequent concurrent presentation of depressive symptoms. Despite these symptoms affecting up to 45% of CAD patients,4 79% of US cardiovascular physicians lack a standard method to screen for them.5 Depressive symptoms are associated with noncompletion of cardiac rehabilitation6 and a 2‐fold increased risk of mortality compared with nondepressed CAD patients.7 Despite their frequent comorbid presentation, there is a lack of knowledge on the underlying biological processes linking CAD and depression.8 As a result, the study of biomarkers could provide additional clinical information with implications for the underlying biological changes associated with depression.
In our present study we investigate the utility of phospholipids in predicting depressive symptoms in CAD patients because altered lipid metabolism may be a fundamental mechanism linking vascular disease and depression.9, 10 Aberrant phospholipid metabolism can affect depressive symptoms through activation of dopamine and serotonin receptors, which are involved in traditional depression pathophysiology.11 More recently, inflammation downstream of phospholipid signaling has been studied at the intersection of CAD and depression.12 Indeed, individual blood‐based biomarkers of inflammation (eg, cytokines) and oxidative stress (eg, malondialdehyde) correlate with depressive symptoms in subsets of patients.13
Briefly, phospholipids comprise a head group and 2 long‐chain fatty acid tails. There are various head groups: phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidylethanolamine, and sphingomyelin (SM), each of which may have a different impact on membrane biology.11 Lipidomics, a subcategory of metabolomics, provides a comprehensive analysis of all phospholipids14 and can be used to define a biosignature of multiple phospholipids associated with depressive symptoms in CAD patients. The use of lipidomics has revealed insights into the differential importance of phospholipid head groups. In a previous study lipidomics showed that mice administered the antidepressants maprotiline and paroxetine had lower PC levels and higher lyso‐PC levels in the prefrontal cortex. This modulatory effect of antidepressants on phospholipids may increase levels of docosahexaenoic acid.15 Although most studies report on the relationship between n‐3 fatty acids in patients with CAD or depression, the relationship between other phospholipids are not as well studied. As a progression from our previous work,10 we expanded our scope to include other phospholipids in addition to those containing n‐3 fatty acids.
We performed a biomarker discovery study using targeted lipidomics, tested our model's ability to identify CAD patients with depressive symptoms using receiver operating curves, and validated it using nested cross‐validation and multivariate regressions. Our primary objective was to establish a phospholipid biomarker panel that can identify CAD patients with significant depressive symptoms. Our secondary objective was to construct a composite biosignature score from the phospholipid biomarker model and compare it between CAD patients demonstrating significant depressive symptoms and CAD controls.
Materials and Methods
The data used in this study will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because of the sensitive nature of patient confidentiality. However, methods and materials used in our analyses are described in detail in the following sections.
Patients
This cross‐sectional study was conducted using baseline data from the CAROTID trial (Coronary Artery Disease Randomized Omega‐3 Trial in Depression, NCT00981383).16 Patients were recruited from a cardiac rehabilitation program at the University Health Network Toronto Rehabilitation Institute. All patients had CAD (myocardial infarction, prior revascularization procedure, and/or angiographic evidence of at least 50% stenosis in a major coronary artery). They were all between the ages of 45 to 80, spoke and understood English, and provided written and informed consent, as described previously.16 Patients were excluded if they had any of the following comorbidities: a history of a central neurological condition, an Axis I psychiatric disorder other than depression, a significant acute medical illness, or a substance abuse disorder. The use of antidepressants was permitted as long as the dose had been stable for at least 3 months before inclusion. The research ethics boards at Sunnybrook Health Sciences Centre and the University Health Network approved this study.
Assessments
Demographic information, medical history, vascular risk factors (hypertension, smoking, hypercholesterolemia, obesity, and diabetes mellitus), measures of cardiopulmonary fitness, and concomitant medications were collected at baseline. Cardiopulmonary fitness was measured by the peak volume of oxygen (VO2 peak) utilized by a patient during an exercise stress test at entry to the cardiac rehabilitation program. Fasting blood samples were collected to determine the erythrocyte percentage composition (RBC%) of phospholipid species. RBC% of various phospholipid species was quantified because it is a stable measure that closely reflects percentage composition of those in neuronal membranes 17 and is not sensitive to daily changes in dietary intake.18
Severity of depressive symptoms was evaluated using the CES‐D (Center for Epidemiologic Studies Depression) scale, which is a 20‐item self‐report questionnaire that assesses positive affect, negative affect, somatic complaints, and interpersonal problems.19 A higher CES‐D score indicates more severe depressive symptoms. A cutoff score of 16 or greater was used to distinguish older adults with significant depressive symptoms from those with insignificant depressive symptoms.20
Phospholipid Analysis
Blood for lipidomics was drawn at each visit at 0900 hours (±30 minutes) to minimize dietary and diurnal influences. Blood was collected in ethylenediaminetetraacetid acid sample tubes, centrifuged at 666 g for 10 minutes, separated, and frozen at −80°C. The percentage of fatty acids in each erythrocyte phospholipid class (PC, phosphatidylethanolamine, PS, PI, SM, and lyso‐PC) was determined by thin‐layer chromatographic separation of phospholipid classes, followed by analysis of fatty acids by gas chromatography as previously described.10 In total, 378 phospholipid species were identified.
Biomarker Discovery and Validation
A biomarker discovery and validation analysis was performed using Metaboanalyst 3.0.21 It is a preferred platform for metabolomic analyses, offering a wide range of statistical methods. There are 4 steps involved: (1) data preprocessing; (2) biomarker selection; (3) model performance evaluation; and (4) phospholipid biosignature assessment. A visual step‐by‐step progression is presented in Figure 1. Additional details are provided in subsequent subsections.
Figure 1.
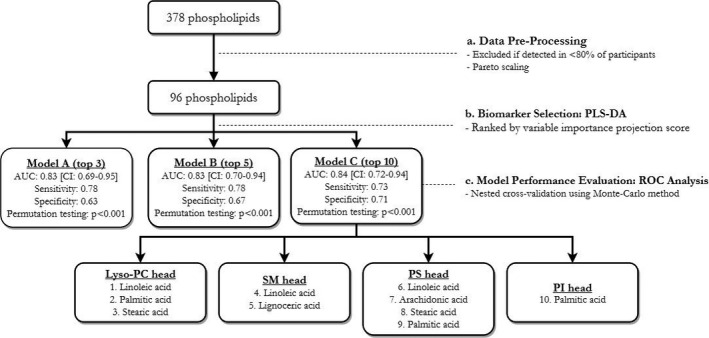
A step‐by‐step progression from all the measured phospholipids to those included in the final biomarker model. AUC indicates area under the curve; CI, confidence interval; lyso‐PC, lyso‐phosphatidylcholine; PC, phosphatidylcholine; PI, phosphatidylinositol; PS, phosphatidylserine; ROC, receiver operating curves; SM, sphingomyelin.
Data Preprocessing
Of 378 phospholipid species, 282 phospholipid species were detected in fewer than 80% of patients due to low concentrations; thus, they were excluded from analyses. Of the remainder, any missing values were replaced by the mean of the corresponding phospholipid in each group. Data were normalized with pareto scaling (mean‐centered and divided by the square root of the SD of each variable) to reduce skewness of data. Out of the 92 patients in the CAROTID study, 6 were excluded from our analyses because they did not have metabolite measurements.
Biomarker Selection: Partial Least‐Squares Discriminant Analysis
Potential phospholipids of interest were selected using partial least‐squares discriminant analysis, a standardized supervised classification technique used for biomarker selection in metabolomic studies.22 Partial least‐squares discriminant analysis was used to extract a subset from 96 phospholipid species via linear combination to predict a maximum separation between patients with significant and nonsignificant depressive symptoms. Potential phospholipid species of interest were selected based on a ranking of their variable importance projection scores.
Model Performance Evaluation: Receiver Operator Characteristic Analysis
The ability of biomarker models to discriminate between groups was evaluated using receiver operator characteristic curve analysis. The performance of each biomarker model was presented as the area under the curve (AUC) and its corresponding 95% confidence interval. A greater AUC value indicates greater sensitivity and specificity of the predictive model.
Three biomarker models, consisting of 3, 5, and 10 phospholipid species, were evaluated. Given that we do not have an independent population to validate the biomarker model, nested cross‐validation using the Monte‐Carlo method was performed to examine the ability of a chosen model to discriminate between depressed and control patients.23 In Monte Carlo cross‐validation the study population is split into a training set (two‐thirds of study population) and a testing set (the remaining one‐third). Then, a biomarker model was constructed using a training set, and the predictive ability of this chosen model was validated using the test set; both sets contained equal representation of patients with and without significant depressive symptoms. This cross‐validation was repeated with random samplings in permutation testing to generate confidence intervals for AUC. Permutation testing was used to examine internal consistency: whether the predictive ability of a selected model was significantly different from that of a randomly generated null model. A resulting empirical P<0.05 means that fewer than 5% of randomly permutated models (n=1000) have a predictive performance similar to that of a selected model.
Statistical Analyses
Statistical analyses were conducted using IBM SPSS Statistics, version 20 (Chicago, IL). Analyses were 2‐tailed, and statistical significance was defined as P<0.05. Student t tests and chi‐squared (χ2) tests were conducted to identify differences in demographic and clinical characteristics between depressed and control groups; any factors identified to be significantly different between groups were adjusted for in subsequent analyses. To ensure that our findings were robust, sensitivity analyses were conducted by including characteristics with P‐values of less than 0.1 in subsequent regression models.
A composite biosignature score was calculated by taking the sum of the standardized RBC% of the 10 phospholipids that were included in the validated biomarker model. Because a single standardized score is generated, this approach avoids the issue of multiple comparisons and presents RBC% of individuals on a comparable scale. Analyses of covariance were performed to see whether biosignature scores were different between those who had significant depressive symptoms and those who did not, after adjustment for potential confounders. Analyses of covariance were also conducted to compare RBC% of individual phospholipid species that made up the biosignature between these 2 cohorts, after adjustment for potential confounders. The relationship between biosignature scores and severity of depressive symptoms using CES‐D scores was evaluated in linear regression models, after adjustment for potential confounders. Linear regressions were also performed to compare differences in the individual phospholipid species that made up the biosignature.
Results
Patient Characteristics
Between August 2010 and February 2014, 86 patients with stable CAD were enrolled and provided samples for the present analysis. The majority of our population was white (72.8%) and male (74.4%). The demographic and clinical characteristics are summarized in Table 1. The CES‐D score indicated that 37 patients (43%) had significant depressive symptoms. This cohort with significant depressive symptoms had significantly lower VO2 peak (t=2.30 [df=84], P<0.005) with an average of 17.2±4.4 mL/(min·kg), compared with 19.9±6.1 mL/(min·kg) in those with no significant depressive symptoms. In terms of medical history, 24 (64.8%) of those with significant depressive symptoms also had a history of depression, which is a significantly greater portion than the 13 (26.5%) of those with no significant depressive symptoms (χ2=12.64 [df=1], P<0.005). Moreover, those with significant depressive symptoms had a greater number of vascular risk factors with an average of 3.7±1.4 compared with an average of 2.5±1.4 in those who did not have significant depressive symptoms (t=−4.01 [df=84], P<0.005). Last, those with significant depressive symptoms were more likely to be taking calcium channel blockers (CCBs) (χ2=6.42 [df=1], P=0.011): 13 (35.1%) of those with depressive symptoms were taking this class of drugs compared with 6 (12.2%) of those without.
Table 1.
Demographic and Clinical Differences Between Coronary Artery Disease Patients With and Without Significant Depressive Symptoms
| Patients Without Significant Depressive Symptoms (n=49) | Patients With Significant Depressive Symptoms (n=37) | t or χ2 (df), P Value | |
|---|---|---|---|
| Male, n (%) | 39 (79.6) | 25 (67.6) | 1.60 (1), 0.210 |
| Age, mean (SD) | 62.8 (8.6) | 60.4 (9.0) | 1.24 (84), 0.220 |
| BMI, mean (SD) | 27.7 (4.7) | 29.6 (5.5) | −1.71 (84), 0.090 |
| VO2 peak, mean (SD) | 19.9 (6.1) | 17.2 (4.4) | 2.30 (84), 0.020a |
| Living alone, n (%) | 5 (10.2) | 9 (24.3) | 3.08 (1), 0.080 |
| History of depression, n (%) | 13 (26.5) | 24 (64.8) | 12.64 (1), <0.005a |
| Antidepressant use, n (%) | 5 (10.2) | 7 (19.4) | 1.10 (1), 0.294 |
| Anxiolytic use, n (%) | 1 (2.0) | 4 (10.8) | 2.96 (1), 0.085 |
| β‐Blockers, n (%) | 36 (73.5) | 24 (64.9) | 0.74 (1), 0.390 |
| ARB, n (%) | 10 (20.4) | 4 (10.8) | 1.43 (1), 0.233 |
| ACE inhibitor, n (%) | 28 (57.1) | 24 (64.9) | 0.53 (1), 0.468 |
| NSAID, n (%) | 43 (87.8) | 30 (81.1) | 0.73 (1), 0.392 |
| Calcium channel blocker, n (%) | 6 (12.2) | 13 (35.1) | 6.42 (1), 0.011a |
| Myocardial infarction, n (%) | 14 (28.6) | 16 (43.2) | 3.11 (3), 0.380 |
| Number of cardiovascular risk factors, mean (SD) | 2.5 (1.4) | 3.7 (1.4) | −4.01 (84), <0.005a |
| sMMSE, mean (SD) | 28.7 (1.1) | 28.4 (1.3) | 1.14 (84), 0.260 |
Denotes variables that were significantly different between groups and used as covariates in subsequent analyses. This is based on statistical significance that is defined by a P value of <0.05.
ACE indicates angiotensin‐converting enzyme blocker; ARB, angiotensin II receptor blocker; BMI, body‐mass index; df, degrees of freedom; NSAID, nonsteroidal anti‐inflammatory drug; sMMSE, standardized Mini‐Mental State Examination scores; VO2 peak, maximal oxygen consumption.
A 10‐Phospholipid Model Identifies Patients With Significant Depressive Symptoms
Receiver operator characteristic analyses revealed that a model composed of 10 phospholipid species had the greatest AUC value compared with those composed of 3 and 5 phospholipid species (Table 2; Figure 2). The 10‐phospholipid model was carried forward through the remainder of results because of its smaller confidence interval around the AUC. The identities of these 10 phospholipid species are presented in Table 3.
Table 2.
Comparison of 3 Classifier Models With 3, 5, and 10 Phospholipids and Their Corresponding Performance (AUC Value, Sensitivity, and Specificity) in Distinguishing Patients With Significant Depressive Symptoms From Those Without
| Number of Phospholipids | AUC [95% CI] | Sensitivity | Specificity | Permutation Testing (n=1000) |
|---|---|---|---|---|
| 3 | 0.83 [0.69 to 0.95] | 0.78 | 0.63 | P<0.001 |
| 5 | 0.83 [0.70 to 0.94] | 0.78 | 0.67 | P<0.001 |
| 10 | 0.84 [0.72 to 0.94] | 0.73 | 0.71 | P<0.001 |
AUC indicates area under the curve CI, confidence interval.
Figure 2.
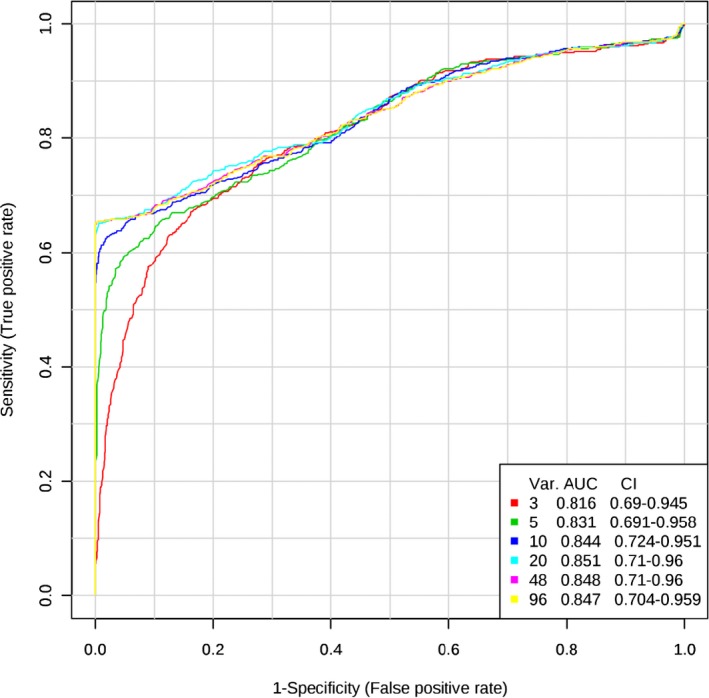
Receiver operating characteristics (ROC) curves compare the area under the curve (AUC) and confidence interval (CI) of classifier models consisting of 3, 5, and 10 phospholipid species (Var). Larger models with 20, 48, and 96 phospholipid species are shown for comparison.
Table 3.
Analyses of Covariance Show Associations Between Standardized Percentage of Individual Phospholipids in Red Blood Cell Membrane From the 10‐Phospholipid Model and Whether Patients Had Significant Depressive Symptoms
| Phospholipids (Head Group) | Mean (SD) in Patients With Nonsignificant Symptoms (n=49) | Mean (SD) in Patients With Significant Depressive Symptoms (n=37) | ANCOVAHa | Linear Regressiona | ||
|---|---|---|---|---|---|---|
| F(dfb), P Value | Unstandardized Coefficient (B) | Standard Error (SE) | P Value | |||
| Linoleic acid (Lyso‐PC) | 0.33 (1.11) | −0.42 (0.63) | 7.95 (1.86), 0.006 | −2.03 | 1.31 | 0.125 |
| Palmitic acid (Lyso‐PC) | −0.29 (0.91) | 0.38 (1.00) | 4.71 (1.86), 0.033 | 1.69 | 1.31 | 0.201 |
| Stearic acid (Lyso‐PC) | −0.24 (0.91) | 0.31 (1.04) | 3.24 (1.86), 0.076 | 2.38 | 1.27 | 0.063 |
| Palmitic acid (PI) | −0.33 (0.91) | 0.44 (0.95) | 9.99 (1.86), 0.002 | 3.14 | 1.27 | 0.015 |
| Linoleic acid (SM) | 0.34 (1.00) | −0.45 (0.82) | 5.70 (1.86), 0.019 | −3.44 | 1.32 | 0.011 |
| Lignoceric acid (SM) | −0.45 (0.62) | 0.60 (1.10) | 22.72 (1.86), <0.0005 | 5.49 | 1.24 | <0.0005 |
| Linoleic acid (PS) | 0.22 (1.05) | −0.29 (0.85) | 4.65 (1.86), 0.034 | −3.98 | 1.21 | 0.001 |
| Arachidonic acid (PS) | −0.40 (0.94) | 0.53 (0.82) | 27.95 (1.86), <0.0005 | 5.29 | 1.18 | <0.0005 |
| Stearic acid (PS) | −0.25 (1.06) | 0.33 (0.81) | 12.51 (1.86), 0.001 | 3.93 | 1.22 | 0.002 |
| Palmitic acid (PS) | 0.36 (1.09) | −0.48 (0.60) | 11.19 (1.86), 0.001 | −3.78 | 1.29 | 0.004 |
Linear regression models show associations between standardized RBC% of individual phospholipids and Centre for Epidemiologic Studies Depression Scale (CES‐D) score.
All analyses adjusted for VO2peak, history of depression, number of vascular risk factors, and use of CCB.
First number indicates degrees of freedom between groups; second number indicates degrees of freedom within groups.
CCB indicates calcium channel blocker; df, degrees of freedom; PC, phosphatidylcholine; PI, phosphatidylinositol; PS, phosphatidylserine; SM, sphingomyelin.
Phospholipid Biosignature Scores are Significantly Higher in Patients With Significant Depressive Symptoms Compared With Those Without
The mean (±SD) composite biosignature scores were 0.42±1.00 in CAD patients with significant depressive symptoms and −0.32±0.89 in CAD controls. Biosignature scores were significantly higher in those with significant depressive symptoms compared with CAD controls after adjustment for VO2 peak, history of depression, number of vascular risk factors, and use of CCB (F[1.86]=14.39, P<0.0005). Of the 10 phospholipid species, RBC% of palmitic acid (PA) with a lyso‐PC head group, PA with a PI head group, arachidonic acid (AA) with a PS head group, lignoceric acid with a SM head group, and stearic acid (SA) with a PS head group were significantly higher in depressed patients, whereas the RBC% of linoleic acid (LA) with lyso‐PC, SM, and PS head groups, and PA with a PS head group were significantly lower in depressed patients. All analyses were adjusted for VO2 peak, history of depression, number of vascular risk factors, and use of CCB (Table 3).
Phospholipid Biosignature Scores Correlated With Severity of Depressive Symptoms
In a linear regression model every unit increase in the biosignature score was associated with a 4.19 increase in CES‐D score after adjustment for VO2 peak, history of depression, number of vascular risk factors, and use of CCB (B=4.19 [SE=1.22], P=0.001). Of the 10 phospholipid species that comprised the biosignature score, lignoceric acid with a SM head group, AA with a PS head group, PA with a PI head group, and SA with a PS head group were individually associated with greater CES‐D scores, whereas LA with SM and PS head groups and PA with a PS head group were individually associated with lower CES‐D scores after adjusting for VO2 peak, history of depression, number of vascular risk factors, and use of CCB (Table 3).
Discussion
Using targeted lipidomics in combination with multivariate analyses, we constructed a model of 10 phospholipids and showed that it can distinguish CAD patients with significant depressive symptoms from those without. The 10 phospholipids include LA in erythrocyte lyso‐PC, SM, and PS; lignoceric acid in erythrocyte SM; AA in erythrocyte PS; PA in erythrocyte lyso‐PC, PI, and PS; and SA in erythrocyte lyso‐PC and PS. This model had good internal validity as seen through its performance in both permutation and cross‐validation testing, which suggested that its performance would be similar in an independent sample. The biosignature scores were significantly higher in the depressed cohort compared with the control cohort, independently of VO2 peak, history of depression, number of vascular risk factors, and use of CCB. The biosignature scores also correlated with symptom severity. The present findings support a growing interest in lipidomic approaches to optimize biomarker identification and warrant further investigation.
Potential Implications of Fatty‐Acid Specificity in a Phospholipid Biomarker Model
The proinflammatory nature of phospholipids in our model supports inflammation as a primary contributor to the progression of depression.24 For example, LA is a polyunsaturated n‐6 essential fatty acid that serves as a precursor to the longer‐chain n‐6 AA.25 Increased concentrations of LA lead to higher inflammatory marker concentrations such as interleukin‐8 and elevated oxidative stress.26 Likewise, high levels of LA have been associated with greater urine levels of prostaglandin products, further supporting its association with inflammation.27 However, in all 3 head groups—lyso‐PC, SM, and PS—our results indicate that depressed patients had lower levels of LA compared with nondepressed patients. These discrepant results may reflect elevated activity of δ‐6 desaturase in patients with significant depressive symptoms, converting more LA to the biologically active AA as previously observed.28 This aligns with our observation that patients with significant depressive symptoms have higher levels of AA compared with the nondepressed population. Given that AA metabolism produces several inflammatory products such as leukotrienes, prostaglandins, and thromboxane A4, the elevation of AA in our depressed cohort supports the association between depression and inflammation.29 Taken together, LA and AA are consistently associated with more severe depressive symptoms in cross‐sectional studies, presenting biological plausibility that they are involved in the mechanistic link between CAD and depressive symptoms. As such, decreasing their levels in the body may provide a therapeutic effect in those with depression. Alternatively, high levels of n‐6 fatty acids may reflect a lack of n‐3 fatty acids in the diet, because both fatty acid families compete for the same rate‐limiting δ‐6 desaturase, which mediates their conversion to their biologically active metabolites.
Saturated fatty acids were consistently elevated in our depressive cohort, which corroborates previous findings that they are a risk factor for depressive symptoms.30 Our results show that levels of the saturated fatty acid, lignoceric acid, were higher in CAD patients with significant depressive symptoms. In comparison, Assies and colleagues found lower levels of lignoceric acid in people with major depressive disorder compared with controls.31 Although its impact on depressive symptoms is inconclusive, 63% of individuals in the study done by Assies and colleagues were taking antidepressants, which could have contributed to normalizing the levels of lignoceric acid. Similarly, CAD patients with significant depressive symptoms had higher levels of another saturated fatty acid, PA in erythrocyte lyso‐PC, PS, and PI. These results are consistent with those of Tsuboi and colleagues, who reported positive correlations between serum PA levels and depressive symptom severity.32 Although the serum fraction reflects recent dietary intake rather than long‐term trends, our replication of this association in a different blood fraction supports the potential pathophysiological importance of PA. Another saturated fatty acid, SA in erythrocyte lyso‐PC and PS was also elevated in our at‐risk cohort. This is not unexpected, because related saturated fatty acids are also upregulated in this population, suggesting a shared mediating factor.
Collectively, saturated fatty acids may precipitate depressive symptoms through inflammation‐mediated mechanisms. For example, PA treatment in adipocytes induced expression of interleukin‐6 and accumulation of reactive oxygen species.33 These inflammatory signals can be transmitted across the blood‐brain barrier by activation of glia cells and cumulate in the release of inflammatory cytokines.34 The resulting neuroinflammation increases oxidative stress, which changes neuronal membrane stability and affects catecholamine neurotransmission, a mechanism involved in traditional depressive pathophysiology.35 Similarly, peripheral SA levels increase brain inflammation through activation of toll‐like receptor 4 in the hypothalamus 36 and may be responsible for dysregulation of the hypothalamus‐pituitary‐adrenal axis, a frequent observation in depressed populations.37 Taken together, we postulate that the association of lignoceric acid, PA, and SA with inflammation may be relevant in the progression of depression in CAD.
Potential Implications of Head Group Specificity in a Phospholipid Biomarker Model
Inter‐head‐group differences in phospholipid levels were not found in erythrocyte PC and PE, the most abundant phospholipid classes.38 Instead, they were identified in erythrocyte PI, SM, PS, and lyso‐PC. The head‐group specificity of these findings may be attributed to the net charge of the head groups. The negative or positive charges of PI, SM, PS, and Lyso‐PC head groups may affect membrane fluidity or allow for interactions with hydrolytic enzymes, which convert these structural phospholipids into regulatory messengers 38 and subsequently influence neurotransmission. In contrast, the neutral charge of PC and PE means that they are unlikely to participate in cellular signaling.
There are a multitude of inflammatory pathways through which phospholipid head groups can affect depressive symptoms in CAD. Phospholipase A2 (PLA2) converts erythrocyte PC and PS into lysophospholipids that readily leave the membrane to function as regulatory messengers.39 Lyso‐PC released from glial cells initiate neurodegenerative processes through inflammatory cascades.40 Likewise, activated platelets, a common feature in CAD, catalyze the deacylation of PS into lyso‐PS.41 In contrast to lyso‐PC, lyso‐PS attenuates expression of inflammatory mediators in atherosclerosis.42 In fact, its levels are positively correlated with blood serotonin43 and have been investigated for its antidepressant effects.44 PI is an important membrane substrate on which kinases act to yield secondary messengers such as PIP2, DAG, and IP3. These secondary messengers ultimately activate proinflammatory cascades that are relevant in cardiovascular disease and affective disorders.45 Although the immune effects of lyso‐PC, PS, and PI are not synergistic, their elevated levels in the depressed cohort suggest the overactivation of PLA2, which has been previously observed in patients with recurrent depression.46 Furthermore, it is interesting to note that most of the identified phospholipids are endogenously synthesized by fatty acid elongases within the same pathways.31 Considering the mutual direction of RBC% difference, this also suggests hyperactivity of fatty acid elongase.
Study Strengths and Limitations
Our exploratory model is robust as it was able to identify those with significant depressive symptoms after Monte Carlo cross‐validation and permutation testing was done to reduce model overfitting. In addition, a composite biosignature score was computed from this model to provide a more stable measurement and to avoid multiple comparisons. This biosignature score differed significantly between groups and correlated with severity of depressive symptoms. Furthermore, our biosignature analyses adjusted for anthropomorphic variables, which have been found to be associated with depressive outcomes.
However, this study did not include a comparative healthy cohort without CAD. Thus, although significant differences in levels of phospholipids between groups may indicate metabolic differences due to depressive pathophysiology, they may also be due to the presence of cardiovascular disease in our clinical population. To address this, our analyses adjusted for the number of vascular risk factors, but this is a surrogate measure of cardiovascular influence as it assumes that each factor confers equal risk. Also, the expression of erythrocyte phospholipids as percentage of RBC membrane composition rather than concentration may restrict generalizability of these findings. These analytical nuances may complicate the applicability of phospholipids as a potential biomarker. We preferentially expressed the phospholipids as RBC%, to normalize their relative abundance, ensuring that more abundant phospholipids do not obscure the impact of less abundant phospholipids. Also, we wanted to understand their relationship relative to the rest of the RBC. Lastly, the use of gas chromatography‐flame ionization detector is limited to the fatty acyl group associated with the phospholipid. In future studies the use of tandem mass spectrometry may improve specificity in identifying individual species.
Although our cross‐sectional analysis is an important first step, the next step is to verify this model in repeated assessments in the same clinical population. The sample size is modest, so it may not represent other CAD populations with depressive symptoms. Future studies should aim to validate these findings in an independent population with a larger sample size. The integration of other biological modalities such as gene and protein expression may improve fit of the proposed model with observed depressive symptoms.
Conclusions
This biomarker discovery study found that a 10‐phospholipid model was able to distinguish between CAD patients with and without significant depressive symptoms. Composite biosignature scores, calculated from this model, were higher in CAD patients with significant depressive symptoms and correlated with severity. These findings support the role of proinflammatory phospholipids in the development of depressive symptoms in CAD patients. The head group specificity of these findings suggests that previous associations of total RBC phospholipids and depression may have neglected other important factors that may also contribute to this relationship. Additional studies to investigate causality will be needed. Integrating other biological modalities such as gene and protein expression may increase fit of the proposed model with the observed depressive symptoms.
Sources of Funding
The authors acknowledge operating funds from the Ontario Mental Health Foundation and the Canadian Institutes of Health Research (CIHR) MOP 114913 to Lanctôt and Herrmann. Mazereeuw received a CIHR Training Program in Neurodegenerative Lipidomics Graduate Scholarship as well as an Ontario Graduate Scholarship. This research was supported by an operating grant from the CIHR Partners as part of the Canadian Consortium on Neurodegeneration in Aging (CCNA) (CIHR Funding Reference Number CAN 137794) (Lanctôt and Herrmann).
Disclosures
None.
(J Am Heart Assoc. 2018;7:e008278 DOI: 10.1161/JAHA.117.008278.)29730646
References
- 1. Unal B, Critchley JA, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation. 2004;109:1101–1107. [DOI] [PubMed] [Google Scholar]
- 2. Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2016;67:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huffman JC, Celano CM, Beach SR, Motiwala SR, Januzzi JL. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feinstein RE, Blumenfield M, Orlowski B, Frishman WH, Ovanessian S. A national survey of cardiovascular physicians’ beliefs and clinical care practices when diagnosing and treating depression in patients with cardiovascular disease. Cardiol Rev. 2006;14:164–169. [DOI] [PubMed] [Google Scholar]
- 6. Caulin‐Glaser T, Maciejewski PK, Snow R, LaLonde M, Mazure C. Depressive symptoms and sex affect completion rates and clinical outcomes in cardiac rehabilitation. Prev Cardiol. 2007;10:15–21. [DOI] [PubMed] [Google Scholar]
- 7. Barth J, Schumacher M, Herrmann‐Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta‐analysis. Psychosom Med. 2004;66:802–813. [DOI] [PubMed] [Google Scholar]
- 8. Lichtman JH, Bigger JT Jr, Blumenthal JA, Frasure‐Smith N, Kaufmann PG, Lespérance F, Mark DB, Sheps DS, Taylor CB, Froelicher ES; American Heart Association Prevention Committee of the Council on Cardiovascular Nursing; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Epidemiology and Prevention; American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research; American Psychiatric Association . Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768–1775. [DOI] [PubMed] [Google Scholar]
- 9. Hibbeln JR, Salem N Jr. Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am J Clin Nutr. 1995;62:1–9. [DOI] [PubMed] [Google Scholar]
- 10. Mazereeuw G, Herrmann N, Ma DW, Hillyer LM, Oh PI, Lanctôt KL. Omega‐3/omega‐6 fatty acid ratios in different phospholipid classes and depressive symptoms in coronary artery disease patients. Brain Behav Immun. 2016;53:54–58. [DOI] [PubMed] [Google Scholar]
- 11. Horrobin DF. Phospholipid metabolism and depression: the possible roles of phospholipase A2 and coenzyme A‐independent transacylase. Hum Psychopharmacol. 2001;16:45–52. [DOI] [PubMed] [Google Scholar]
- 12. Shimbo D, Chaplin W, Crossman D, Haas D, Davidson KW. Role of depression and inflammation in incident coronary heart disease events. Am J Cardiol. 2005;96:1016–1021. [DOI] [PubMed] [Google Scholar]
- 13. Lopresti AL, Maker GL, Hood SD, Drummond PD. A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:102–111. [DOI] [PubMed] [Google Scholar]
- 14. Postle AD. Lipidomics. Curr Opin Clin Nutr Metab Care. 2012;15:127–133. [DOI] [PubMed] [Google Scholar]
- 15. Lee LH, Shui G, Farooqui AA, Wenk MR, Tan CH, Ong WY. Lipidomic analyses of the mouse brain after antidepressant treatment: evidence for endogenous release of long‐chain fatty acids? Int J Neuropsychopharmacol. 2009;12:953–964. [DOI] [PubMed] [Google Scholar]
- 16. Mazereeuw G, Herrmann N, Xu H, Blanchard AP, Figeys D, Oh PI, Bennett SA, Lanctôt KL. Platelet activating factors are associated with depressive symptoms in coronary artery disease patients: a hypothesis‐generating study. Neuropsychiatr Dis Treat. 2015;11:2309–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Assies J, Lieverse R, Vreken P, Wanders RJ, Dingemans PM, Linszen DH. Significantly reduced docosahexaenoic and docosapentaenoic acid concentrations in erythrocyte membranes from schizophrenic patients compared with a carefully matched control group. Biol Psychiatry. 2001;49:510–522. [DOI] [PubMed] [Google Scholar]
- 18. Franco RS. Measurement of red cell lifespan and aging. Transfus Med Hemother. 2012;39:302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shafer AB. Meta‐analysis of the factor structures of four depression questionnaires: Beck, CES‐D, Hamilton, and Zung. J Clin Psychol. 2006;62:123–146. [DOI] [PubMed] [Google Scholar]
- 20. Lyness JM, Cox C, Curry J, Conwell Y, King DA, Caine ED. Older age and the underreporting of depressive symptoms. J Am Geriatr Soc. 1995;43:216–221. [DOI] [PubMed] [Google Scholar]
- 21. Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res. 2015;43:W251–W257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gromski PS, Muhamadali H, Ellis DI, Xu Y, Correa E, Turner ML, Goodacre R. A tutorial review: metabolomics and partial least squares‐discriminant analysis—a marriage of convenience or a shotgun wedding. Anal Chim Acta. 2015;879:10–23. [DOI] [PubMed] [Google Scholar]
- 23. Xia J, Broadhurst DI, Wilson M, Wishart DS. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics. 2013;9:280–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alashmali SM, Hopperton KE, Bazinet RP. Lowering dietary n‐6 polyunsaturated fatty acids: interaction with brain arachidonic and docosahexaenoic acids. Curr Opin Lipidol. 2016;27:54–66. [DOI] [PubMed] [Google Scholar]
- 26. Young VM, Toborek M, Yang F, McClain CJ, Hennig B. Effect of linoleic acid on endothelial cell inflammatory mediators. Metabolism. 1998;47:566–572. [DOI] [PubMed] [Google Scholar]
- 27. Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega‐6 polyunsaturated fatty acids. J Nutr Metab. 2012;2012:539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cribb L, Murphy J, Froud A, Oliver G, Bousman CA, Ng CH, Sarris J. Erythrocyte polyunsaturated fatty acid composition is associated with depression and FADS genotype in Caucasians. Nutr Neurosci. 2017. Available at: https://www.tandfonline.com/doi/abs/10.1080/1028415X.2017.1327685?journalCode=ynns20. Accessed April 24, 2018. [DOI] [PubMed] [Google Scholar]
- 29. Samuelsson B. Arachidonic acid metabolism: role in inflammation. Z Rheumatol. 1991;50(suppl 1):3–6. [PubMed] [Google Scholar]
- 30. Sanchez‐Villegas A, Verberne L, De Irala J, Ruiz‐Canela M, Toledo E, Serra‐Majem L, Martínez‐González MA. Dietary fat intake and the risk of depression: the SUN Project. PLoS One. 2011;6:e16268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Assies J, Pouwer F, Lok A, Mocking RJ, Bockting CL, Visser I, Abeling NG, Duran M, Schene AH. Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case‐control study. PLoS One. 2010;5:e10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsuboi H, Watanabe M, Kobayashi F, Kimura K, Kinae N. Associations of depressive symptoms with serum proportions of palmitic and arachidonic acids, and α‐tocopherol effects among male population—a preliminary study. Clin Nutr. 2013;32:289–93. [DOI] [PubMed] [Google Scholar]
- 33. Davis JE, Gabler NK, Walker‐Daniels J, Spurlock ME. The c‐Jun N‐terminal kinase mediates the induction of oxidative stress and insulin resistance by palmitate and toll‐like receptor 2 and 4 ligands in 3T3‐L1 adipocytes. Horm Metab Res. 2009;41:523–530. [DOI] [PubMed] [Google Scholar]
- 34. Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257:139–155. [DOI] [PubMed] [Google Scholar]
- 35. Su KP. Biological mechanism of antidepressant effect of omega‐3 fatty acids: how does fish oil act as a ‘mind‐body interface’? Neurosignals. 2009;17:144–152. [DOI] [PubMed] [Google Scholar]
- 36. Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Varghese FP, Brown ES. The hypothalamic‐pituitary‐adrenal axis in major depressive disorder: a brief primer for primary care physicians. Prim Care Companion J Clin Psychiatry. 2001;3:151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kay JG, Grinstein S. Phosphatidylserine‐mediated cellular signaling. Adv Exp Med Biol. 2013;991:177–193. [DOI] [PubMed] [Google Scholar]
- 39. Meyer zu Heringdorf D, Himmel HM, Jakobs KH. Sphingosylphosphorylcholine‐biological functions and mechanisms of action. Biochim Biophys Acta. 2002;1582:178–189. [DOI] [PubMed] [Google Scholar]
- 40. Sundaram JR, Chan ES, Poore CP, Pareek TK, Cheong WF, Shui G, Tang N, Low CM, Wenk MR, Kesavapany S. Cdk5/p25‐induced cytosolic PLA2‐mediated lysophosphatidylcholine production regulates neuroinflammation and triggers neurodegeneration. J Neurosci. 2012;32:1020–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sato T, Aoki J, Nagai Y, Dohmae N, Takio K, Doi T, Arai H, Inoue K. Serine phospholipid‐specific phospholipase A that is secreted from activated platelets. A new member of the lipase family. J Biol Chem. 1997;272:2192–2198. [DOI] [PubMed] [Google Scholar]
- 42. Nishikawa M, Kurano M, Ikeda H, Aoki J, Yatomi Y. Lysophosphatidylserine has bilateral effects on macrophages in the pathogenesis of atherosclerosis. J Atheroscler Thromb. 2015;22:518–26. [DOI] [PubMed] [Google Scholar]
- 43. Kurano M, Dohi T, Nojiri T, Kobayashi T, Hirowatari Y, Inoue A, Kano K, Matsumoto H, Igarashi K, Nishikawa M, Miyauchi K, Daida H, Ikeda H, Aoki J, Yatomi Y. Blood levels of serotonin are specifically correlated with plasma lysophosphatidylserine among the glycero‐lysophospholipids. BBA Clin. 2015;4:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Komori T. The effects of phosphatidylserine and omega‐3 fatty acid‐containing supplement on late life depression. Ment Illn. 2015;7:5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cantrell DA. Phosphoinositide 3‐kinase signalling pathways. J Cell Sci. 2001;114:1439–1445. [DOI] [PubMed] [Google Scholar]
- 46. Galecki P, Galecka E, Maes M, Chamielec M, Orzechowska A, Bobinska K, Lewiński A, Szemraj J. The expression of genes encoding for COX‐2, MPO, iNOS, and sPLA2‐IIA in patients with recurrent depressive disorder. J Affect Disord. 2012;138:360–366. [DOI] [PubMed] [Google Scholar]


