Figure 3. ZIP14 is upregulated in cachectic muscles from metastatic mouse models and patients, and is induced by TNF-α and TGF-β.
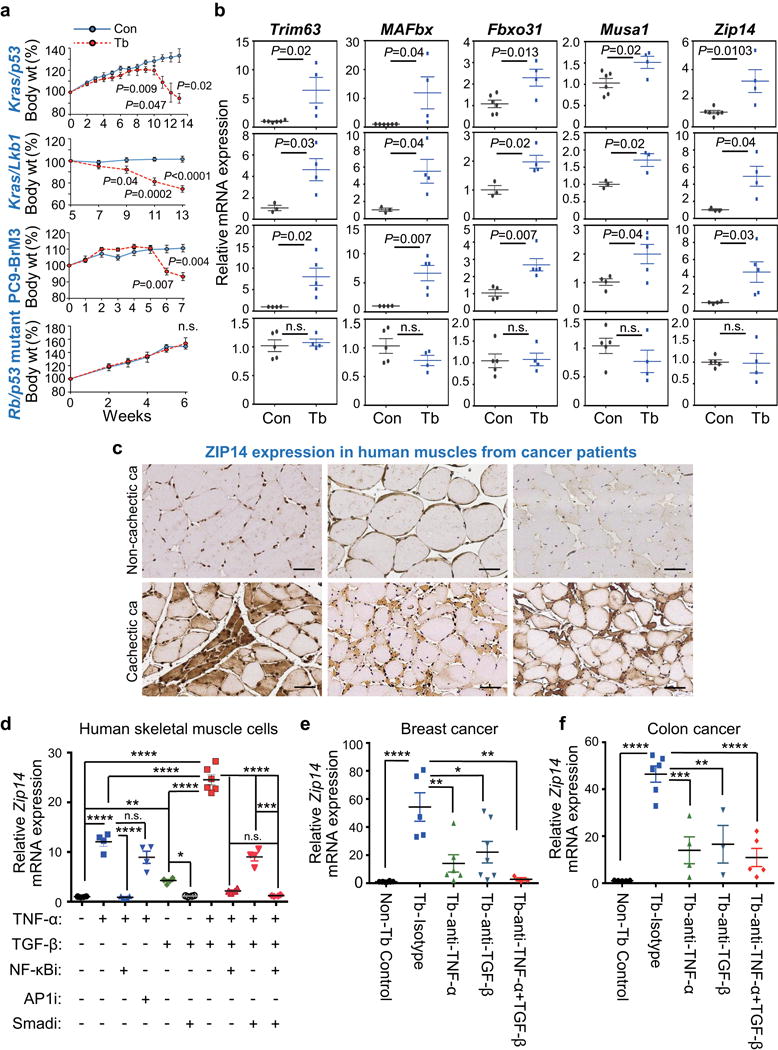
(a,b) Body weight measurements (a) and qRT-PCR analysis of Trim63, MAFbx, Fbxo31, Musa1 and Zip14 in TA muscles (b) derived from four independent metastatic lung cancer models, compared to respective age-matched controls. Metastatic models include conditional Kras/p53 mutant (KrasLSL-G12D/+-p53fl/fl) and Kras/Lkb1 mutant (KrasLSL-G12D/+-Lkb1fl/fl) in which muscles were collected at 13 weeks post adeno-Cre induction, PC9-BrM3 xenograft in which muscles were collected at 7 weeks post tumor-cell injection, and Rb/p53 mutant allografts in which muscles were collected at 6 weeks post tumor resection (see also Supplementary Fig. 3a). For body weight analysis in (a), n=4 controls and n=8 Kras/p53 conditional mutant mice, n=3 controls and n=6 Kras/Lkb1 conditional mutant mice, n=3 controls and n=10 PC9-BrM3 xenograft mice, and n=3 controls and n=10 Rb/p53 mutant allograft mice. For qRT-PCR analysis in (b), n=6 controls and n=4 Kras/p53 conditional mutant mice, n=3 controls and n=4 Kras/Lkb1 conditional mutant mice, n=4 controls and n=5 PC9-BrM3 xenograft mice, and n=5 controls and n=4 Rb/p53 mutant allograft model mice.
(c) Representative images of ZIP14 immunohistochemistry on human muscle cross-sections from non-cachectic (upper panel) and cachectic (lower panel) metastatic cancer patients. ZIP14 antibody details are shown in Supplementary Fig. 3f-h. Scale bars, 50 μm. ca, cancer. Patient details are listed in Supplementary Table 3. Data is representative of three independent experiments.
(d) qRT-PCR analysis of Zip14 in human skeletal primary muscle cells treated with either vehicle, TNF-α (50 ng/ml), TGF-β (10 ng/ml), or both TNF-α (50 ng/ml) + TGF-β (10 ng/ml), either alone (vehicle) or in the presence of 10 μM of the indicated inhibitors. Cells were pretreated with either vehicle or the indicated inhibitors for 1 hour prior to adding the cytokines (TGF-β for 9 hours, and TNF-α for 3 hours before harvest). Cells from all groups were harvested at the same time. n=6 samples for cells treated with vehicle, TGF-β alone or TNF-α together with TGFβ; n=4 samples for all the other groups. Inhibitors: NF-κBi = NF-κB inhibitor (BAY 11-7085); AP1i = AP1 inhibitor (CC401); Smadi = TGFβ receptor I inhibitor (SB431542).
(e,f) qRT-PCR analysis of Zip14 in TA muscles after neutralizing antibody treatment. Following the tumor-resection-and-relapse approach (see Fig. 1a and Supplementary Fig. 1c), mice injected with either 4T1 (e) or C26m2 tumor cells (f) were treated with either an isotype control antibody, or a neutralizing antibody against TNF-α, TGF-β, or both (200 μg antibody per mouse treated three times a week) starting one week after surgery, for a period of one week. For 4T1 model, n=8 non-tumor bearing control mice, n=5 tumor bearing mice treated with isotype control, n=6 tumor bearing mice treated with TNF-α antibody, n=7 tumor bearing mice treated with TGF-β antibody, and n=3 tumor bearing mice treated with both TNF-α and TGF-β antibodies. For C26m2 model, n=5 non-tumor bearing control mice, n=6 tumor bearing mice treated with isotype control, n=4 tumor bearing mice treated with TNF-α antibody, n=3 tumor bearing mice treated with TGF-β antibody, and n=5 tumor bearing mice treated with both TNF-α and TGF-β antibodies.
Error bars represent SEM and all data were represented by mean ± SEM. P values were determined by two-tailed, unpaired Student’s t test in (a, b) and with one-way ANOVA with post-hoc Tukey’s test in (d-f) with F value (DFn, DFb) for d F(9, 36) = 161.9, e F(4, 24) = 10.3, and f F(4, 18) = 20.7. n.s., not significant. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. Con, non-tumor bearing control; Tb, tumor-bearing.
