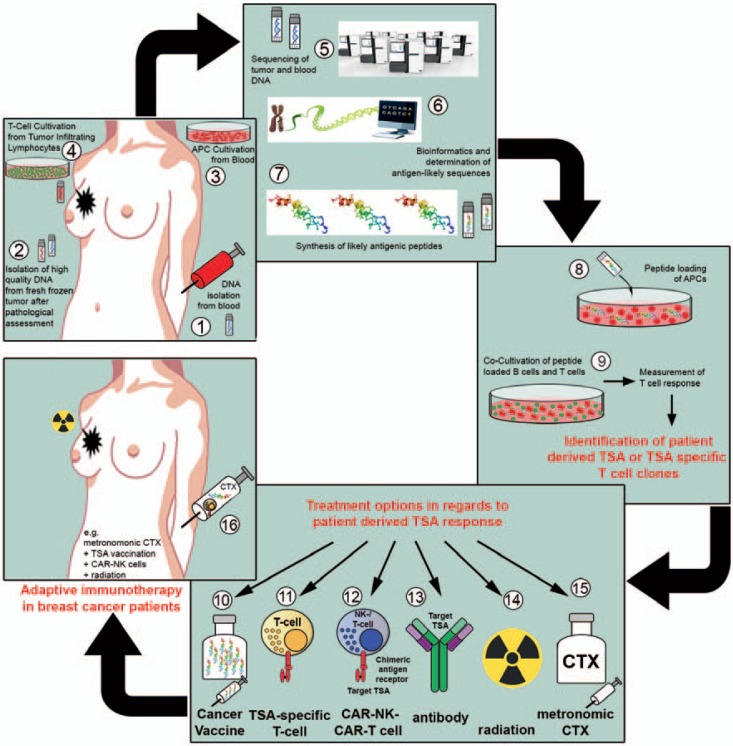
Fig. 4.
Objective and strategy of the TILGen study and therapy concept. The overall objective of the TILGen study is the identification of tumor-specific antigens (TSAs)/neoepitopes as well as TSA-specific T cells, and the implementation of TSA/neoepitope-specific targeted therapy. The workflow with each working step is illustrated in numbers (1–16): 1) blood sample collection for isolation of germline DNA; 2) collection of fresh frozen breast cancer core biopsies for isolation of tumor DNA and RNA; 3) isolation and cultivation of antigen-presenting cells (APCs); 4) T-cell expansion out of tumor-infiltrating lymphocytes (TILs) from core biopsy; 5) whole genome sequencing; 6) determination of likely antigenic sequences by comparing germline and tumor DNA; 7) synthesis of likely antigenic peptides; 8) loading of antigenic peptides on APCs; 9) co-cultivation of peptide-loaded APCs and isolated tumor T cells to measure T cell response and identify TSAs and TSA-specific T cell clones; 10) production of cancer vaccine with regard to TSA; 11) expansion of TSA-specific T cells; 12) production of chimeric antigen receptor (CAR) natural killer cells (NK) or CAR T cells; 13) production of TSA-specific humanized antibodies; 14) low-dose radiation therapy; 15) metronomic chemotherapy; 16) treatment option with e.g. metronomic chemotherapy in combination with TSA vaccination, TSA-specific T cells, and radiation.