FIG 1 .
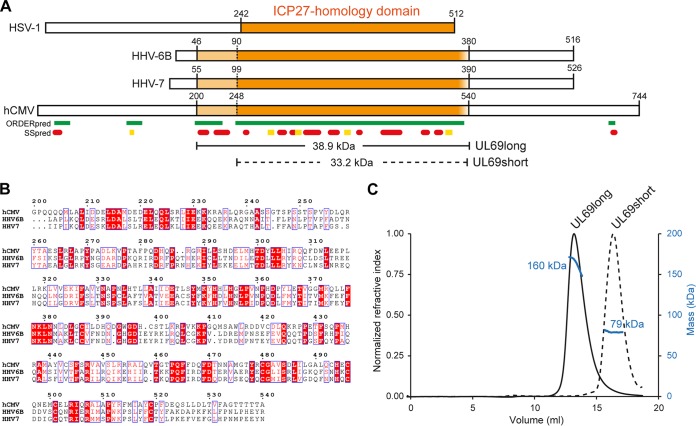
HCMV UL69 self-associates via a conserved ICP27 homology domain and forms a tetramer. (A) Location of the ICP27 homology domain (IHD) (orange) with the prototype from HSV-1 and the homologues from HHV-6A, HHV-6B, and HCMV. Prediction within HCMV UL69 of ordered/structured regions is indicated by green blocks, and the secondary structure is shown by red cylinders for α-helices and yellow blocks for β-sheets (29). The domain boundaries for constructs used here are indicated along with the molecular weight based on the primary sequence (30). (B) Primary sequence alignment of IHDs from UL69 homologues within the betaherpesvirus subfamily, produced with ESPrint (35). (c) SEC-MALS analysis of the molecular weights of purified UL69 constructs indicates that tetramer formation within UL69long is destabilized to a dimer by truncation of the region from aa 200 to 247 in UL69short.