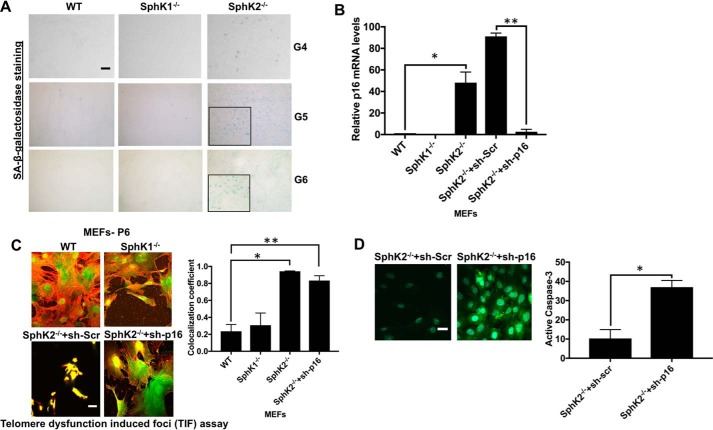
Figure 8.
Genetic loss of SphK2 results in accelerated p16-dependent senescence but not apoptosis via increased telomere damage in noncancerous fibroblasts. A, senescence in testes tissues obtained from WT and SphK2−/− mice at generations 4–6 was measured by SA-β-gal staining. Scale bars represent 100 μm. B, abundance of p16 mRNA was measured by qPCR in MEFs isolated from WT, SphK1−/−, and SphK2−/− mice at generation 6 in the absence/presence of Scr- or p16-shRNAs. Data are means ± S.D. from three independent experiments, analyzed by Student's t test (n = 3, *, p = 0.0402; **, p = 0.0019). C and D, effects of p16 knockdown on telomere damage (C). Data are means ± S.D. from three independent experiments, analyzed by Student's t test (n = 3, *, p = 0.006; **, p = 0.013) or caspase-3 activation (D), and were measured using immunofluorescence in MEFs obtained from WT, SphK1−/−, and SphK2−/− mice at generation 6. Scr-shRNA transfected SphK2−/− MEFs were used as controls. Images were quantified using ImageJ (right panels). Data are means ± S.D. from three independent experiments, analyzed by Student's t test (n = 3, *, p = 0.001).