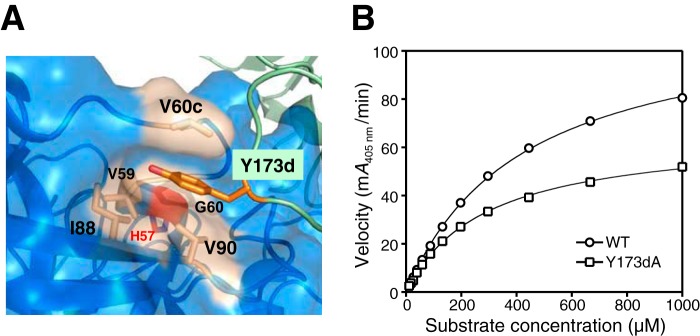
Figure 11.
Characterization of the Y173dA tetramer mutant. A, Y173d from one protomer in the large interface lies in a hydrophobic pocket of its neighboring protomer. It is sandwiched between Val-90 and Val-60c with surrounding Val-59, Gly-60, and Ile-88 hydrophobic residues; the 60s loop is relatively close to the catalytic His-57 (red). B, dependence of reaction velocity on S-2288 substrate for tetrameric WT β-tryptase and the Y173dA mutant. Data were collected in triplicate and fit to the Michaelis-Menten equation; errors are shown as S.D.