Figure 7.
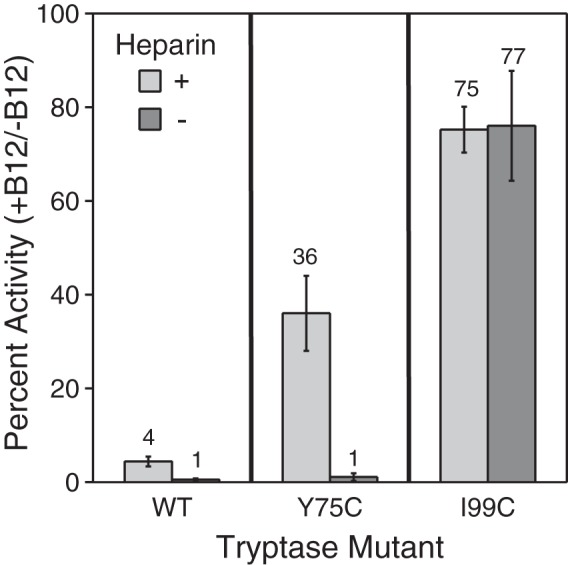
Activity of tryptase WT and disulfide-locked dimer mutants after dissociation with B12 Fab. The percent activity remaining of WT, Y75C, and I99C tryptase mutants (1 nm) in the presence of B12 Fab (125 nm) compared with its absence was determined in the presence (light gray) and absence (dark gray) of 0.1 mg/ml heparin. Tryptase variants were incubated with B12 Fab for 15 min at room temperature prior to activity measurements. The percent activity for each tryptase variant in the absence of B12 Fab and the presence and absence of 0.1 mg/ml heparin is defined as 100%. Data were collected in triplicate for two independent determinations; errors are shown as S.D.
