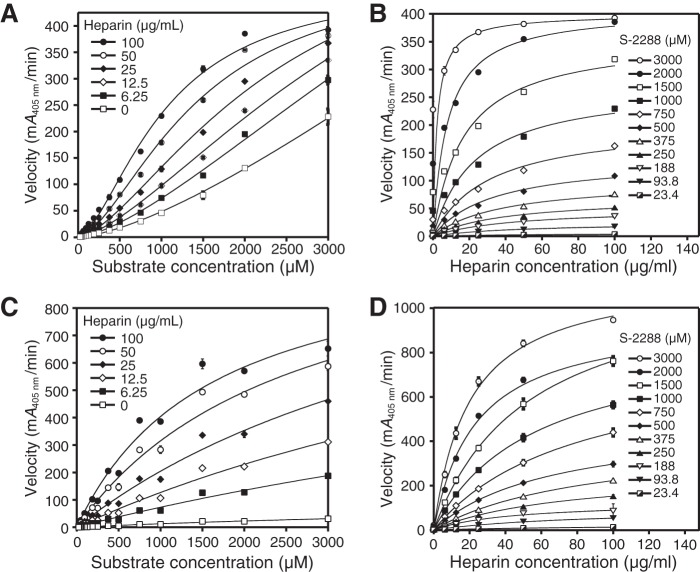
Figure 9.
Dependence of velocity on substrate and heparin for activated dimeric I99C/Y75A/Y37bA and monomeric I99C*/Y75A/Y37bA β-tryptase mutants. A, activated I99C/Y75A/Y37bA dimer data for the dependence of velocity with 2 mm S-2288 substrate at different heparin concentrations are shown. Data were collected in triplicate and fit to a sigmoidal equation. B, activated I99C/Y75A/Y37bA dimer data for the dependence of velocity on heparin at different S-2288 substrate concentrations are shown. C, activated I99C*/Y75A/Y37bA monomer data for the dependence of velocity on S-2288 substrate at different heparin concentrations are shown. D, activated I99C*:Y75A:Y37bA monomer data for the dependence of velocity on heparin at different S-2288 substrate concentrations are shown. Data for B, C, and D were each collected in triplicate and fit to hyperbolic binding equations and the 1:1 binding isotherm for B and D or Michaelis-Menten equation for C. Errors in all data are shown as S.D. Tryptase dimer and monomer concentrations were 100 and 400 nm, respectively.