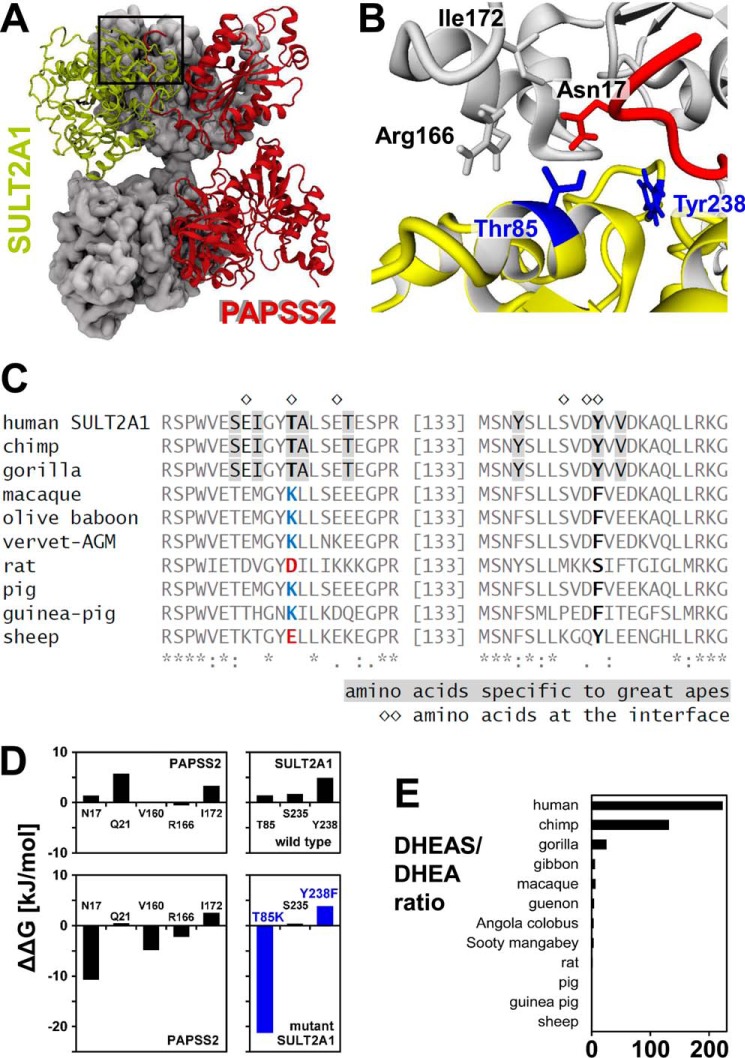
Figure 7.
A PAPSS2–SULT2A1 protein interaction facilitates DHEA sulfation. A and B, molecular representation of a PAPSS2–SULT2A1 complex averaged over 15 ns of MD time. The dimeric PAPSS2 subunit proximal to SULT2A1 is depicted in gray color and with molecular surface representation; the distant PAPSS2 subunit in red color and ribbon representation. SULT2A1 is drawn in yellow. Please note the composite nature of the PAPSS2-binding site. Amino acids on the interface are shown in stick representation of the side chains and labeled accordingly. C, all SULT2A1 amino acids on the interface with PAPSS2 were highlighted in an alignment of diverse mammalian SULT2A1 protein sequences. The only two interface amino acids that were specific to great apes are Thr85 and Tyr238 (depicted in blue in B). D, the PAPSS2–SULT2A1 interface was analyzed using Rosetta-based alanine scanning (27). Furthermore, the two great ape-specific amino acids were mutated to their nonhominoid counterparts. T85K resulted in a dramatic loss of stability of the complex. E, the hominid-specific PAPSS2–SULT2A1 complex coincides with a higher DHEAS/DHEA ratio in gorilla, chimpanzee, and human. DHEAS/DHEA ratios are derived from Refs. 28 and 29.