Abstract
The sphingolipid ceramide is not only a precursor of more complex sphingolipids, but also a potent signaling molecule. Specific ceramide species have distinct cellular functions, and each ceramide synthase therefore has particular roles in cells and organisms. Tidhar and colleagues, utilizing two ceramide synthases differing widely in fatty acid specificity, have identified a short amino acid sequence that is critical for this specificity. This work represents a crucial first step in the understanding of both the enzymology and the biology driving the diverse functions of ceramide.
Introduction
Lipid biochemists often introduce their work by citing the enormous diversity of lipid species, 40,000 or more, that are found in eukaryotic cells. This metric begs two questions: How is this diversity generated, and what functions are served by it? Answering the second question depends on understanding the first. Tidhar et al. (1) have now addressed how molecular diversity is generated for ceramide, a bioactive sphingolipid class that encompasses almost 200 different molecular species. Their work significantly advances our understanding of how ceramide synthases, ceramide-producing enzymes that are embedded in the membrane of the endoplasmic reticulum (ER), generate a spectrum of ceramide species.
It has now become apparent that the biological activities of ceramides depend on their molecular species. Traditionally, ceramide was broadly characterized as a pro-apoptotic cellular lipid. Indeed, in many cases, it has been well established that elevated ceramide levels trigger apoptosis (2). However, recent findings have prompted a more nuanced view. For example, in cells derived from squamous cell carcinoma, C16-ceramide was reported to be anti-apoptotic and C18-ceramide pro-apoptotic (3), whereas in other settings, C16-ceramide is pro-apoptotic (4, 5). Similarly, very-long-chain ceramides induce mitochondrial dysfunction and cell death in cardiomyocytes, whereas long-chain (C16 and C18) ceramides do not (6). In addition, specific ceramide molecular species have critical functions in differentiated tissues such as the skin and brain (reviewed in Ref. 7). In light of the diverse and indispensable roles of ceramides in many organisms, the work by Tidhar et al. (1) unraveling the molecular mechanism underlying ceramide synthase specificity for different acyl-CoAs represents an important milestone toward elucidating and manipulating ceramide activities in the cell.
Sphingolipids such as ceramide comprise a metabolically interrelated group of lipids built on the sphingosine backbone, and their de novo biosynthetic pathway is relatively straightforward. The initiating step is the condensation of serine and the 16-carbon fatty acid palmitate (conjugated to CoA) to produce 3-ketodihydrosphingosine, which is then reduced to dihydrosphingosine (also known as sphinganine). At this point, the ceramide synthases N-acylate dihydrosphingosine, utilizing acyl-CoAs of varying chain lengths. The resultant dihydroceramides are then converted to ceramides by a desaturase that introduces a 4,5-trans-double bond into the sphingosine backbone. Ceramide synthases can also acylate sphingosine to produce ceramide by a salvage pathway.
Mammals have six ceramide synthases (CerS1–6),2 each exhibiting a preference for the chain length of the fatty acyl-CoA substrate and producing a distinct ceramide species. For example, CerS5 strongly prefers palmitoyl-CoA, a 16-carbon fatty acyl-CoA, and thus predominantly produces C16-ceramide. In contrast, CerS2 favors long-chain fatty acyl-CoAs and produces ceramides incorporating 22–24-carbon fatty acids. Because no crystal structure of any ceramide synthase is available, and their topology is ambiguous, Tidhar et al. (1) set out to define sequences in the ceramide synthases that determine fatty acid preferences by exploiting the differential specificity between CerS2 and CerS5.
Using chimeras of CerS2 and CerS5, the authors had previously identified a 150-residue region that dictated this specificity (8). This region contains several membrane-anchoring domains and hydrophilic loops. The challenge was to identify the specific site(s) defining substrate specificity within this relatively large segment of the protein. They identified two ancient frameshifts in the nucleotide sequences. These sequences encode two short amino acid segments, one in putative transmembrane segment 2 and the other in a hydrophilic loop, presumably in the lumen, between the final two putative transmembrane segments. Considering the hydrophobic nature of the fatty acyl-CoA substrate, the transmembrane segment seemed the more likely determining sequence. However, introducing the CerS2 sequence at that site into the CerS5 backbone did not result in any change in substrate preference.
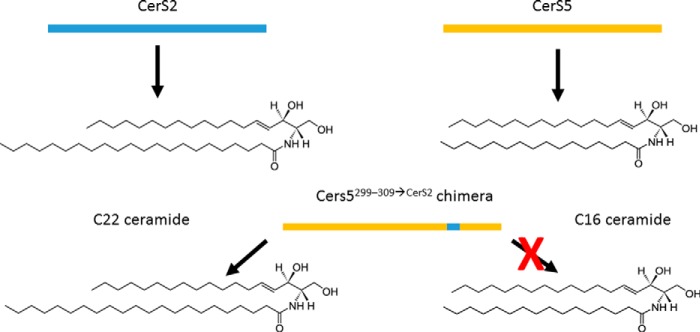
The authors therefore tested the role of the hydrophilic loop, predicted to be in the lumen of the ER and close to the C terminus of the protein. When an 11-amino acid-long sequence derived from CerS2 (which uses C22–24 fatty acyl-CoAs as substrates) was inserted into the backbone of CerS5 (which uses C16 fatty acyl-CoA as substrate) at that site, the overexpressed enzyme had CerS2-like activity, efficiently using C22 fatty acyl-CoA as a substrate (Fig. 1). These results demonstrated that this hydrophilic loop is a critical component of substrate specificity of CerS2 and CerS5. For the first time, there is now a structural correlate to the substrate specificity, and therefore biological impact, of individual ceramide synthases. Another important advance reported by the authors, using an endogenous glycosylation site, is that there is an odd number of transmembrane segments, most probably five, with additional membrane-anchoring domains that do not traverse the membrane.
Figure 1.
An 11-residue segment in ceramide synthases determines substrate (and product) specificity. When 11 amino acids in a loop located between the last two putative transmembrane domains from CerS2 replace the corresponding residues in CerS5, the chimeric protein CerS5299–309→CerS2 produces C22-ceramide (a typical CerS2 product) instead of the C16-ceramide that CerS5 normally produces.
This study represents an important step in the detailed understanding of the structural enzymology behind substrate selection in the ceramide synthases, but significant work remains to be done. Clearly, a high-resolution molecular structure of a ceramide synthase will be required for a complete understanding of the substrate selectivity in this enzyme family. The recent acceleration in the determination of membrane protein structures suggests that determining the structure of a ceramide synthase may soon be within reach. For example, the structural features determining fatty acyl-CoA selectivity of a membrane-embedded protein acyltransferase were recently revealed by X-ray crystallography (9).
Layered on the selectivity encoded within the ceramide synthase proteins are the cellular conditions that determine the levels and delivery mechanisms of the fatty acyl-CoAs, which also dictate the levels of the ceramide molecular species. An intriguing recent observation is that an acyl-CoA carrier protein can stimulate CerS2 and CerS3 activities, and the levels of this carrier protein can have a critical impact on the generation of very-long-chain ceramides (10).
We are just beginning to understand the individual roles of the six mammalian ceramide synthases and to define the precise activities of the distinct ceramide molecular species that they produce. Determining how ceramides exert their effects has been a thorny problem. Ceramides profoundly affect the physical properties of membrane lipids and thereby impact membrane protein function and membrane permeability. Moreover, ceramides can directly bind to and affect the function of some proteins. Elucidating ceramide activities and discovering small molecules that can modulate those functions in therapeutic settings will require the ability to precisely manipulate the molecular species produced. The work of Tidhar et al. (1) provides a significant advance in this direction.
This work was supported by National Institutes of Health Grant 1R01HL131340. The author declares that he has no conflicts of interest with the contents of this article. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
- CerS
- ceramide synthase
- ER
- endoplasmic reticulum.
References
- 1. Tidhar R., Zelnik I. D., Volpert G., Ben-Dor S., Kelly S., Merrill A. H. Jr, and Futerman A. H. (2018) Eleven residues determine the acyl chain specificity of ceramide synthases. J. Biol. Chem. 293, 9912–9921 10.1074/jbc.RA118.001936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hannun Y. A., and Obeid L. M. (2002) The ceramide-centric universe of lipid-mediated cell regulation: Stress encounters of the lipid kind. J. Biol. Chem. 277, 25847–25850 10.1074/jbc.R200008200 [DOI] [PubMed] [Google Scholar]
- 3. Senkal C. E., Ponnusamy S., Manevich Y., Meyers-Needham M., Saddoughi S. A., Mukhopadyay A., Dent P., Bielawski J., and Ogretmen B. (2011) Alteration of ceramide synthase 6/C16-ceramide induces activating transcription factor 6-mediated endoplasmic reticulum (ER) stress and apoptosis via perturbation of cellular Ca2+ and ER/Golgi membrane network. J. Biol. Chem. 286, 42446–42458 10.1074/jbc.M111.287383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mesicek J., Lee H., Feldman T., Jiang X., Skobeleva A., Berdyshev E. V., Haimovitz-Friedman A., Fuks Z., and Kolesnick R. (2010) Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 22, 1300–1307 10.1016/j.cellsig.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sassa T., Suto S., Okayasu Y., and Kihara A. (2012) A shift in sphingolipid composition from C24 to C16 increases susceptibility to apoptosis in HeLa cells. Biochim. Biophys. Acta 1821, 1031–1037 10.1016/j.bbalip.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 6. Law B. A., Liao X., Moore K. S., Southard A., Roddy P., Ji R., Szulc Z., Bielawska A., Schulze P. C., and Cowart L. A. (2018) Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J. 32, 1403–1416 10.1096/fj.201700300R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park J. W., Park W. J., and Futerman A. H. (2014) Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim. Biophys. Acta 1841, 671–681 10.1016/j.bbalip.2013.08.019 [DOI] [PubMed] [Google Scholar]
- 8. Tidhar R., Ben-Dor S., Wang E., Kelly S., Merrill A. H. Jr., and Futerman A. H. (2012) Acyl chain specificity of ceramide synthases is determined within a region of 150 residues in the Tram-Lag-CLN8 (TLC) domain. J. Biol. Chem. 287, 3197–3206 10.1074/jbc.M111.280271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rana M. S., Kumar P., Lee C. J., Verardi R., Rajashankar K. R., and Banerjee A. (2018) Fatty acyl recognition and transfer by an integral membrane S-acyltransferase. Science 359, eaao6326 10.1126/science.aao6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferreira N. S., Engelsby H., Neess D., Kelly S. L., Volpert G., Merrill A. H., Futerman A. H., and Færgeman N. J. (2017) Regulation of very-long acyl chain ceramide synthesis by acyl-CoA-binding protein. J. Biol. Chem. 292, 7588–7597 10.1074/jbc.M117.785345 [DOI] [PMC free article] [PubMed] [Google Scholar]