Figure 6.
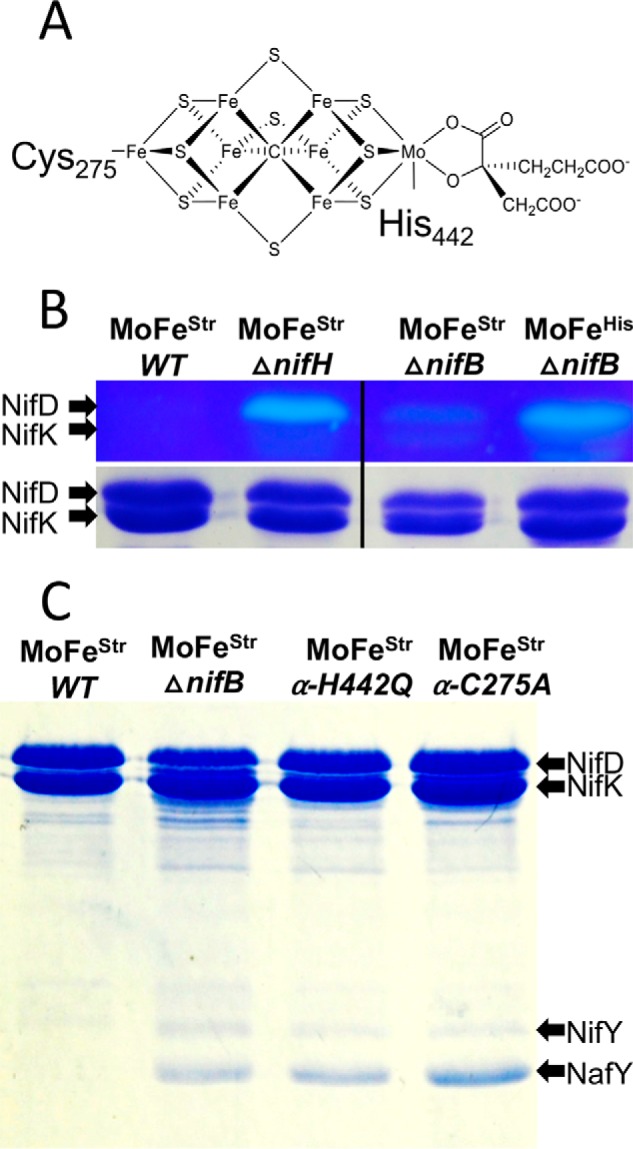
Susceptibility of the α-Cys275 residue of MoFe protein produced in different genetic backgrounds and co-purification of NifY and NafY with MoFe protein having Ala or Gln substituted for either α-Cys275 or α-His442, respectively. A, structure of FeMo-cofactor showing anchoring α-Cys275 and α-His442 residues. B (top), MoFe protein samples purified from different genetic backgrounds and treated with the fluorescent alkylating reagent I-AEDANS before SDS-PAGE and visualized by UV light illumination before Coomassie Brilliant Blue staining (18). Note that B is a composite of a single gel for which a lane located between the ΔnifH sample and ΔnifB sample has been excised. B (bottom), same samples as shown in the top after staining with Coomassie Brilliant Blue. Identities of affinity tags used to assist purification, Strep-tag or His-tag, are indicated by superscripts. C, co-purification of NifY and NafY with Strep-tag affinity-purified MoFe protein produced in different genetic backgrounds.
