Abstract
A serious concern is arising on the coexistence of extended-spectrum beta-lactamase (ESBL) and plasmid mediated quinolone resistance (PMQR) producing bacteria in animal husbandry, which could be transferred to humans, especially in strains that may not be routinely screened for resistance. This study therefore tested the prevalence of ESBL and PMQR genes in selected bacteria isolated from poultry faeces. Faecal droppings of birds were collected from 11 farms in five states in South Western Nigeria. Bacteria were isolated from the samples on cefotaxime supplemented plates and identified with MALDI-TOF. The MIC was determined using VITEK system and resistance genes were detected with PCR. A total of 350 strains were isolated from different samples and selected strains were identified as 23 Klebsiella pneumonia, 12 Morganella morganii, seven Leclercia adecarboxylata and one Citrobacter freundii. All the species were resistant to gentamycin, trimethoprim/sulphamethaxole, tobramycin, piperacillin, cefotaxime and aztreonam (except Morganella morganii strains which were mostly susceptible to aztreonam). All the tested strains were susceptible to imipenem, meropenem and amikacin. All Leclercia adecarboxylata strains were resistant to ceftazidime, cefepime and fosfomycin while all Morganella morganii strains were resistant to fosfomycin, moxifloxacin and ciprofloxacin. All tested species were generally sensitive to ciprofloxacin except Morganella morganii strains which were resistant to ciprofloxacin. The resistance to ciprofloxacin, ceftazidime, cefepime, tigercylin, colistin and fosfomycin were 65%, 40%, 23%,, 7%, 33%, 48% respectively while the prevalence of SHV, TEM and CTX genes were 42%, 63%, 35% respectively. 9.3% of the isolates had the three ESBL genes, 2.33% had qnrA gene, 4.65% had qnr B gene while none had qnrS gene. The most prevalent PMQR gene is Oqxb (25.58%) while 6.98% had the qep gene. Klebsiella pneumoniae generally had both ESBL and PMQR genes. The high prevalence of extended spectrum beta-lactamase genes in the studied strains calls for caution in the use of beta lactam antibiotics in poultry feeds. This is the first report of the occurrence of extended spectrum beta-lactamase and plasmid mediated quinolone resistance genes in Morganella morganii and Leclercia adecarboxylata strains isolated from poultry faeces.
Keywords: Resistance genes, Chicken, Susceptibility, Antibiotics, Pathogens
Introduction
The use of sub-therapeutic antibiotics as growth promoters in poultry feeds in some countries has led to increasing rate of resistance to antibiotics among pathogens in poultry environment. This is enhanced by the ability of the resistant strains to transfer acquired resistance to their progeny and other unrelated bacteria through plasmids (Apata, 2009). Resistance is common to the most frequently prescribed antibiotics such as the quinolones, beta-lactam antibiotics and aminoglycosides. This is observed in different types of infections caused by Gram-negative bacteria, especially Enterobacteriaceae, which may be difficult to treat (Schultsz & Gerlings, 2012). Of more concern are quinolones and beta-lactam antibiotics, which are commonly prescribed in human to treat different infections.
Resistance to quinolones varies across microorganisms and geographical locations. There seems to be a linkage between resistance to quinolones and the β-lactam antibiotics as ESBL genes are frequently carried on plasmids which can also carry genes encoding resistance to other antibiotics e.g., aminoglycosides thereby giving ESBL producers the characteristic broad antibiotic resistance to multiple antibiotic classes (Rawat & Nair, 2010). ESBLs are most commonly found in Enterobacteriaceae and one of the commonest ESBL producers is Escherichia coli (Mansouri & Ramazanzadeh, 2009). They could also occur in uncommon species of Enterobacteriaceae e.g., Morganella morganii, Leclercia adecarboxylata and Citrobacter freundii. Morganella morganii could cause nosocomial and opportunistic infections in intensive care unit patients and immunocompromised hosts (Singla et al., 2010). It has been reported to cause fatal infections in chicken (Zhao et al., 2012). Leclercia adecarboxylata is another rare Enterobacteriaceae isolated from water, which could act as an opportunistic pathogen in immunocompromised patients. It is usually susceptible to most commonly used antibiotics including beta-lactams. However, a few cases of antibiotic-resistant L. adecarboxylata have been reported (Shin et al., 2012). Citrobacter freundii is resistant to β-lactam antibiotics due to the production of ESBL in some strains (Kanamori et al., 2011).
Escherichia coli and Salmonella spp. are often isolated in poultry and its environment as ESBL producers (Saliu, Vahjen & Zentek, 2017) while E. coli and Enterobacter cloacae were the most frequently identified organisms from hatching eggs (Mezhoud et al., 2016). There are information on ESBL-producing E. coli strains from poultry, but little information is available on detection of ESBL-producing Klebsiella pneumonia, Morganella morganii, Leclercia adecarboxylata and Citrobacter freundii in poultry. We have previously studied susceptibility of non E. coli enterobacteriaceae isolated from poultry in Ibadan, Nigeria but the strains were not identified to species level, neither were resistant genes investigated (Ayeni, Olujobi & Alabi 2015). The aim of this study was therefore to determine the presence of ESBL and PMQR resistance genes in Klebsiella pneumonia, Morganella morganii, Leclercia adecarboxylata and Citrobacter freundii strains isolated from faecal dropping of birds in 11 poultry farms from South Western Nigeria.
Materials and Methods
Isolation procedures
The samples were collected between September 2015 and February 2016. A total of 240 fecal droppings were collected aseptically from 11 commercial poultry farms from five states in South Western Nigeria i.e., Oyo, Ondo, Ogun, Ekiti and Osun. Immediately after collection, 1g of each fecal sample was homogenized in 9 mL of MacConkey broth (Oxoid, Cheshire, UK) supplemented with cefotaxime (2 µg/mL) and incubated at 37 °C for 24 h. The incubated broth was plated on MacConkey agar plates (Oxoid, Cheshire, UK) supplemented with cefotaxime (2 µg/mL) (Tamang et al., 2013) at appropriate dilutions and incubated at 37 °C for 24 h. All possible distinct colonies with different morphology that grew on MacConkey plates were sub cultured to get pure cultures which were stored appropriately.
Identification of isolates
Overnight cultures of bacterial isolates were identified with MALDI-TOF apparatus (VITEK® MS (Biomerieux, Nuertingen, Germany)) according to the manufacturer’s instructions. In summary, bacterial strains were grown on MacConkey agar plates for 24 h. A thin smear of the strains were made on MALDI plate and overlaid with 1 µL of matrix solution (saturated solution of α-cyano-4-hydroxycinnamic acid in 50% acetonitrile and 2.5% trifluoroacetic acid) and air dried at room temperature. The plates were put in VITEK® MS (Biomerieux, Nuertingen, Germany) where the organisms were identified by comparing their mass spectra with reference spectra of the manufacturer database. Data were interpreted with scores of ≥2 considered as reliable species level identification. All identified Klebsiella pneumonia, Morganella morganii, Leclercia adecarboxylata and Citrobacter freundii were selected for further studies.
Antibiotic susceptibility of isolates
Bacterial isolates were cultured on MacConkey agar plates and incubated overnight aerobically. Appropriate dilutions of the colonies were made and put into VITEK apparatus for MIC evaluation. The MIC values of different classes of antibiotics were determined by VITEK®2 compact system (AST-N248 cards; Biomerieux, Nuertingen, Germany) according to the manufacturer’s instructions.
Detection of ESBL and PMQR genes
All the isolates were subcultured on MacConkey agar and incubated for 24 hrs. Two colonies of grown bacteria were put in an eppendorf tube containing 1 mL of molecular grade water and mixed with the aid of a vortex mixer. The mixture was boiled for 10 min in a water bath at 100 °C and afterwards centrifuged for 5 min at 1,000 rpm. The supernatant (containing the DNA) was removed carefully using a micropipette without disturbing the pellet. The DNA was stored at −20 °C for PCR analysis.
The primers and PCR conditions used in this study are shown in Table 1. The primers were synthesized by Inqaba Biotechnical Industries (Pty) Ltd, Hatfield, South Africa. Positive and negative control from our laboratory were used. All PCR mixture contained the master mix (half of the total reaction volume), primers (1% of the final volume of the supermix), molecular graded water and 1 µL of the DNA template. The final reaction volume was 20 µl. The PCR product was thereafter viewed by gel electrophoresis after staining in ethidium bromide. A band corresponding to the expected size and positive control was assessed as positive.
Table 1. Primers used for gene detection.
| Gene | Primers | Primer sequence | Annealing temp. | Product size | References |
|---|---|---|---|---|---|
| qnrA | Qnr(A)-F | 5-ATTTCTCACGCCAGGATTTG-3 | 53 °C | 516 bp | Wang et al. (2009) |
| Qnr(A)-R | 5-GATCGGCAAAGGTTAGGTCA-3 | ||||
| qnrB | Qnr(B)-F | 5-GATCGTGAAAGCCAGAAAGG-3 | 53 °C | 469 bp | Wang et al. (2009) |
| Qnr(B)-R | 5-ACGATGCCTGGTAGTTGTCC-3 | ||||
| qnrS | Qnr(S)-F | 5-ACGACATTCGTCAACTGCAA-3 | 53 °C | 417 bp | Wang et al. (2009) |
| Qnr(S)-R | 5-TAAATTGGCACCCTGTAGGC-3 | ||||
| qepA | Qep(A)-F | 5-CTTCTCTGGATCCTGGACAT-3 | 53 °C | 720 bp | Şahinturk et al. (2016) |
| Qep(A)-R | 5-TGAAGATGTAGACGCCGAAC-3 | ||||
| oqxB | Oqx(B)-F | 5-ATCGGTATCTTCCAGTCACC-3 | 56 °C | 541 bp | Şahinturk et al. (2016) |
| Oqx(B)-R | 5-ACTGTTTGTAGAACTGGCCG-3 | ||||
| SHV | SHV-F | 5-TCGCCTGTGTATTATCTCCC-3 | 50 °C | 768 bp | Maynard et al. (2003) |
| SHV-R | 5-CGCAGATAAATCACCACAATG-3 | ||||
| TEM | TEM-F | 5-GAGTATTCAACATTTTCGT-3 | 50 °C | 857 bp | Maynard et al. (2003) |
| TEM-R | 5-ACCAATGCTTAATCAGTGA-3 | ||||
| CTX-M | CTX-F | 5-TTTGCGATGTGCAGTACCAGT AA-3 | 56 °C | 543 bp | Edelstein et al. (2003) |
| CTX-R | 5-CGATACGTTGGTGGTGCCATA-3 |
Results
A total of 350 strains were isolated from different samples and identified. The prevalence of the studied enterobacteriaceae were 7% Klebsiella pneumonia (23 strains isolated from four farms), 3% Morganella morganii (12 strains isolated from one farm), 2% Leclercia adecarboxylata (seven strains isolated from two farms) and 0.3% Citrobacter freundii. The remaining 308 strains were strains of E. coli and Enterobacter cloaceae.
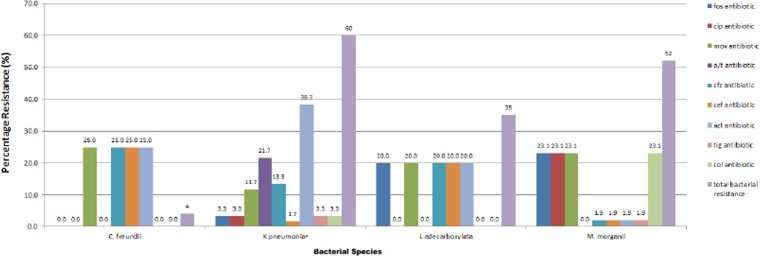
All the four species were resistant to gentamycin, trimethoprim/sulphamethaxole, tobramycin, piperacillin, cefotaxime and aztreonam (except Morganella morganii, which were mostly susceptible to aztreonam). All the strains were sensitive to imipenem, meropenem and amikacin. Most of the strains were sensitive to tigercycline. All Leclercia adecarboxylata strains were resistant to moxifloxacin, ceftazidime, cefepime and fosfomycin. All Morganella morganii strains were resistant to colistin, fosfomycin, moxifloxacin and ciprofloxacin. The only Citrobacter freundii strain had additional resistance to ceftazidime and cefepime. The tested species were mostly sensitive to ciprofloxacin but all Morganella morganii strains were resistant to ciprofloxacin. 65% of the isolates were resistant to ciprofloxacin, 40% were resistant to ceftazidime, 23% were resistant to cefepime, 7% were resistant to tigercylin, 33% were resistant to colistin while 48% were resistant to fosfomycin (Fig. 1).
Figure 1. Resistance profile of bacterial strains.
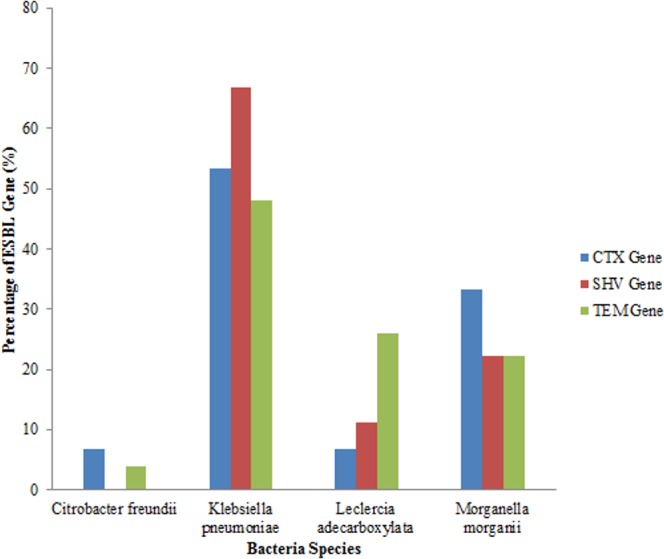
An analysis of all the isolates revealed that 18 (41.86%) had the SHV gene out of which 12 (66.67%) were Klebsiella pneumoniae, (four) 22.22% were Morganella morganii and two (11.11%) were Leclerchia adecarboxylata. Twenty seven (62.79%) of the total isolates had the TEM gene out of which 13 (48.14%) were Klebsiella pneumoniae, 7 (25.93%) were Leclerchia adecarboxylata, six (22.22%) were Morganella morganii and 1 strain of Citrobacter freundii. Also, 15 (34.88%) of the total isolates had the CTX-M gene with 8 (53.33%) being Klebsiella pneumoniae, one (6.67%) was Leclerchia adecarboxylata, 5 (33.33%) were Morganella morganii and 6.67% was one isolate of Citrobacter freundii. Fifteen (34.88%) isolates had both the SHV and TEM genes while four (9.3%) isolates had the three genes and seven (16.28%) isolates had none of the genes. All the Leclerchia adecarboxylata species had the SHV gene while Klebsiella pneumoniae had more SHV and TEM genes than CTX-M gene (Fig. 2).
Figure 2. Distribution of ESBL genes in bacterial strains.
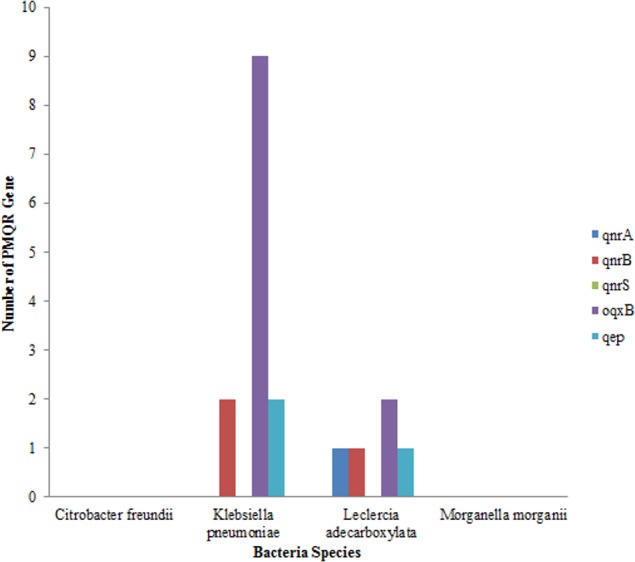
Out of the total of 43 isolates, qnr A gene was only detected in one isolate of Leclerchia adecarboxylata, only two isolates of Klebsiella pneumoniae had the qnr B, gene while no isolate had the qnr S gene. The most prevalent PMQR gene was Oqxb in 11 strains (nine isolates of Klebsiella pneumoniae and two isolates of Leclerchia adecarboxylata). In addition, two isolates of Klebsiella pneumoniae and one isolate of Leclerchia adecarboxylata had the qep gene. None of the isolates had all the PMQR genes (Fig. 3).
Figure 3. Distributions of PMQR among bacterial strains.
Discussion
Antibiotic resistance has become a menace in the environment, as it has rendered the use of several antibiotics in the treatment of certain infections ineffective. A high prevalence of resistant bacteria genes in poultry environment may increase the risk of spread to humans, as the poultry environment is constantly in contact with humans. Observed total resistance of all studied strains to cefotaxime, piperacillin, tobramycin, trimethoprim/sulfamethoxazole and gentamicin conforms to the study by Bradford (2001), in which it was reported that β-lactam antibiotics such as ceftazidime, cefotaxime and oxyimino-monobactam were ineffective against studied bacteria however, carbapenems and cephamycine were effective against ESBL producing strains (Seija et al., 2015). This was also observed in this study, with total susceptibility of all tested organisms to amikacin, imipenem and meropenem. Carbapenems are effective in the treatment of infections caused by multi-drug resistant Gram-negative bacteria including those that produce ESBLs (Walsh et al., 2005). All Morganella morganii and Leclercia adecarboxylata strains in this study were resistant to fosfomycin. It has also been previously noted that ESBL producing isolates displayed higher fosfomycin resistance than ESBL negative strains (Seija et al., 2015). The total resistance of Morganella morganii strains to fosfomycin, colistin and ciprofloxacin is alarming. Demir & Buyukguclu (2013) has also previously reported resistance of all Morganella morganii strains from urogenital infections to fosfomycin and Arca, Reguera & Hardisson (1997) has also reported incorporation of alpha-glycerolphosphate in a fosfomycin resistant Morganella morgannii strain. However, Seija et al. (2015) have successfully reported treatment of multidrug resistant Morganella morganii in sepsis with fosfomycin thereby suggesting that the resistance is not intrinsic. Resistance of Morganella morganii to colistin has been described as intrinsic (Liu et al., 2016).
TEM gene was the most prevalent ESBL gene detected followed by SHV and CTX-M gene in clinical isolates (Machado et al., 2007; Sedighi et al., 2017). Fortini et al. (2011) has also reported dominance of bla (TEM) gene in Enterobacteriaceae isolated from Nigeria and only one strain was positive for the CTX-M gene. However, more prevalence of SHV gene has been reported in isolates from humans in Brazil (Dropa et al., 2009; Naim, Saeed & Malik, 2018). Prevalence of 96.7% ESBL has been previously reported in Klebsiella pneumonia isolated from commercial broiler slaughter plant in Shandong province of China (Wu et al., 2016). This is comparable with the results obtained in this study.
There seems to be a linkage between resistance to quinolones and the β-lactam antibiotics. It has been demonstrated that fluoroquinolone resistance can be mediated by co-transfer of the qnr determinant on ESBL-carrying plasmids (Wang et al., 2004) In some enterobacteriaceae, quinolone resistance is more frequent in ESBL positive stains than in ESBL-negative strains which may be due to combined effects of several mechanisms of resistance (Martinez-Martinez et al., 2002). Cremets et al. (2011) also noted that a CTX-M-15 producing isolate was highly resistant to fluoroquinolones and harboured mutations in the QRDR with two PMQR determinants.
Klebsiella pneumoniae strains had the highest level of resistance to antibiotics, which can be related to the presence of both ESBL and PMQR resistance genes. The most prevalent PMQR gene is oqxB with more abundance in Klebsiella pneumonia strains. (Yuan et al., 2012) and Szabó et al. (2018) also reported the presence of oqxAB gene in all human K. pneumoniae strains investigated. However, in a recent study on Klebsiella strains isolated from broiler, qnrB was the most dominant followed by qnrS and with low prevalence of qepA and qnrA (Wu et al., 2016).
In Morganella morganii, the prevalence of the three ESBL genes was relatively high with the TEM gene being the most prevalent. Al-Muhanna, Al-Muhanna & Alzuhari (2016) also showed that all Morganella morganii isolates tested for the ESBL had all the genes. Morganella morganii produces an inducible, chromosomally encoded AmpC β-lactamse, which can be implicated for its natural resistance to aminoopenicillins, amoxicillin-clavulanate, first and second generation cephalosporins with aztreonam, carbenicillin, and tazobactam being effective transient inactivators of some variants (Power et al., 2006). This is observed in this study, with most Morganella morganii being susceptible to aztreonam. Additionally, Morganella morganii is naturally resistant to tetracyclines, tigectycline, polymyxins and nitrofurantoin (Leclercq et al., 2013). However, intermediate resistance to tigercycline was observed in this study. Although high resistance to ciprofloxacin was observed in Morganella morganii in this study, they didn’t possess any of the PMQR genes investigated. The observed resistance may be due to an univestigated gene. Resistance of Morganella morganii to quinolones has been linked to qnrD, two new gyrB mutations (S463A, S464Y) and one parC mutation (S80I) (Nasri et al., 2014; Szabó et al., 2018).
All the Leclercia adecarboxylata species had the TEM gene, which may be responsible for their complete resistance to ceftazidime and cefotaxime. There was a moderate prevalence of SHV and CTX-M genes. Garcia-Fulgueiras et al. (2014) also reported the first presence of SHV and TEM genes in Leclercia adecarboxylata. The first case of CTX-M in Leclercia adecarboxylata was reported in a multi-drug resistant strain harbouring CTX-M and TEM gene (Shin et al., 2012). The prevalence of qep and oqxB gene was low in Leclercia adecarboxylata. In addition, three Morganella morganii and one Leclercia adecarboxylata had all three (SHV, TEM and CTX-M) genes; this increases the probability of these organisms developing resistance to quinolones.
Limitation of the study
The observed resistance to ciprofloxacin in Morganella morganii strains could be due to qnr D gene. However, the gene was not investigated in this study.
Conclusion
This study reports high prevalence of oqxB, TEM and SHV in poultry faecal dropping. To the best of our knowledge, this is the first study reporting occurrence of ESBL and PMQR in Morganella morganii and Leclercia adecarboxylata isolated from poultry.
Supplemental Information
Acknowledgments
We thank all staff of Institute of Medical Microbiology, Justus Liebig University for identification and antibiotic susceptibility determination of the strains.
Funding Statement
FA Ayeni is a recipient of a TWAS-DFG postdoctoral fellowship at Institute of Medical Microbiology, Justus Liebig University, Giessen, Austria. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Olajumoke R. Akinbami performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper.
Samson Olofinsae performed the experiments, contributed reagents/materials/analysis tools, approved the final draft.
Funmilola A. Ayeni conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.
References
- Al-Muhanna, Al-Muhanna & Alzuhari (2016).Al-Muhanna AS, Al-Muhanna S, Alzuhari MA. Molecular investigation of extended-spectrum beta-lactamase genes and potential drug resistance in clinical isolates of Morganella morganii. Clinical Infectious Diseases. 2016;36(3):223–228. doi: 10.5144/0256-4947.2016.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apata (2009).Apata DF. Antibiotic resistance in poultry. International Journal of Poultry Science. 2009;8(4):404–408. doi: 10.3923/ijps.2009.404.408. [DOI] [Google Scholar]
- Arca, Reguera & Hardisson (1997).Arca P, Reguera G, Hardisson C. Plasmid-encoded fosfomycin resistance in bacteria isolated from the urinary tract in a multicentre survey. Journal of Antimicrobial Chemotherapy. 1997;40(3):393–399. doi: 10.1093/jac/40.3.393. [DOI] [PubMed] [Google Scholar]
- Ayeni, Olujobi & Alabi (2015).Ayeni FA, Olujobi OF, Alabi OS. A preliminary investigation of prevalence of extended spectrum beta lactamases among enterobacteriaceae isolated from poultry farms in Ibadan, Nigeria. Nigerian Journal of Pharmaceutical Research. 2015;11(1):46–51. [Google Scholar]
- Bradford (2001).Bradford PA. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology and detection of this important resistance threat. Clinical Microbiology Reviews. 2001;14(4):933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremets et al. (2011).Cremets L, Caroff N, Dauvergne S, Reynaud A, Lepelleter D, Convec S. Prevalence of plasmid mediated quinolone resistance in ESBL enterobacteriaceae clinical isolates over a 1-year period in a French hospital. Pathologie Biologie. 2011;59(3):151–156. doi: 10.1016/j.patbio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Demir & Buyukguclu (2013).Demir T, Buyukguclu T. Evaluation of the in vitro activity of fosfomycin tromethamine against Gram-negative bacterial strains recovered from community- and hospital-acquired urinary tract infections in Turkey. International Journal of Infectious Diseases. 2013;17(11):e966–e970. doi: 10.1016/j.ijid.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Dropa et al. (2009).Dropa M, Balsalobre LC, Lancopan N, Mamizuka EM, Murakami T, Cassetari VC, Franco F, Guida SM, Balabaas AJ, Passadre LF, Santos SR, Matte GR, Matte MH. Extended spectrum beta lactamases among enterobacteriaceae isolated in a public hospital in Brazil. Revista do Instituto de Medicina Tropical de Sao Paulo. 2009;51(4):203–209. doi: 10.1590/S0036-46652009000400005. [DOI] [PubMed] [Google Scholar]
- Edelstein et al. (2003).Edelstein M, Pimkin M, Edelstein I, Stratchounski L. Prevalence and molecular epidemiology of CTX-M extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrobial Agents and Chemotherapy. 2003;47:3724–3732. doi: 10.1128/AAC.47.12.3724-3732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini et al. (2011).Fortini D, Fashae K, García-Fernández A, Villa L, Carattoli A. Plasmid-mediated quinolone resistance and β-lactamases in Escherichia coli from healthy animals from Nigeria. Journal of Antimicrobial Chemotherapy. 2011;66(6):1269–1272. doi: 10.1093/jac/dkr085. [DOI] [PubMed] [Google Scholar]
- Garcia-Fulgueiras et al. (2014).Garcia-Fulgueiras N, Seijh V, Aguerrebe P, Cordeiro NF, Vignoli R. First report of Leclercia adecarboxylata harbouring multiple resistance genes in Uruguay and review of the literature. Journal of Global Antimicrobial Resistance. 2014;2(2):77–78. doi: 10.1016/j.jgar.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Kanamori et al. (2011).Kanamori H, Yano H, Hirakata Y, Endo S, Arai K, Ogawa M, Shimojima M, Aoyagi T, Hatta M, Yamada M, Nishimaki K, Kitagawa M, Kunishima H, Kaku M. High prevalence of the extended spectrum β-lactamases and the QNR determinants in the Citrobacter species from Japan: the dissemination of CTX- M-2. Journal of Antimicrobial Chemotherapy. 2011;66(10):2255–2262. doi: 10.1093/jac/dkr283. [DOI] [PubMed] [Google Scholar]
- Leclercq et al. (2013).Leclercq R, Cantón R, Brown DFG, Giske CG, Heisig P, MacGowan AP, Mouton JW, Nordmann P, Rodloff AC, Rossolini GM, Soussy CJ, Steinbakk M, Winstanley TG, Kahlmeter G. EUCAST expert rules in antimicrobial susceptibility testing. Clinical Microbiology and Infection. 2013;19:141–160. doi: 10.1111/j.1469-0691.2011.03703.x. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2016).Liu H, Zhu J, Hu Q, Rao X. Morganella morganii a non-negligent opportunistic pathogen. International Journal of Infectious Diseases. 2016;50:10–17. doi: 10.1016/j.ijid.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Machado et al. (2007).Machado E, Coque TM, Canton R, Novaris A, Sousa JC, Baquero F, Poixe L. Portugese Resistant study group. High density of extended spectrum beta lactamase among clinical isolates of enterobacteriaceae from Portugal. Journal of Antimicrobial Chemotherapy. 2007;60(6):1370–1374. doi: 10.1093/jac/dkm381. [DOI] [PubMed] [Google Scholar]
- Mansouri & Ramazanzadeh (2009).Mansouri R, Ramazanzadeh R. Spread of extended-spectrum beta-lactamase producing Escherichia coli clinical isolates in sanandaj hospitals. Journal of Biological Sciences. 2009;9:362–366. doi: 10.3923/jbs.2009.362.366. [DOI] [Google Scholar]
- Martinez-Martinez et al. (2002).Martinez-Martinez L, Pascual A, Conejo Mdel C, Garcia I, Joyanes P, Domenech-Sanchez A, Benedi VJ. Energy-dependent accumulation of norfloxacin and porin expression in clinical isolates of Klebsiella pneumoniae and relationship to extended-spectrum beta-lactamase production. Antimicrobial Agents and Chemotherapy. 2002;46:3926–3932. doi: 10.1128/AAC.46.12.3926-3932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard et al. (2003).Maynard C, Fairbrother JM, Bekal S, Sanschagrin F, Levesque RC, Brousseau R, Masson L, Lariviere S, Harel J. Antimicrobial resistance genes in enterotoxigenic Escherichia coli O149:K91 isolates obtained over a 23-year period from pigs. Antimicrobial Agents and Chemotherapy. 2003;47(10):3214–3221. doi: 10.1128/AAC.47.10.3214-3221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezhoud et al. (2016).Mezhoud H, Chantziaras I, Iguer-Ouada M, Moula N, Garmyn A, Martel A, Smet AT, Haesebrouck F, Boyen F. Presence of antimicrobial resistance in coliform bacteria from hatching broiler eggs with emphasis on ESBL/AmpC-producing bacteria. Avian Pathology. 2016;45(4):493–500. doi: 10.1080/03079457.2016.1167837. [DOI] [PubMed] [Google Scholar]
- Naim, Saeed & Malik (2018).Naim A, Saeed A, Malik TA. Molecular Detection of TEM, SHV and CTX-M genes among gram negative Klebsiella isolates. Current Drug Delivery. 2018;15(3):417–423. doi: 10.2174/1567201815666180101160108. [DOI] [PubMed] [Google Scholar]
- Nasri et al. (2014).Nasri YM, Denden RI, Guo Q, Mastouri M, Aouni M, Wang M. Type II and type IV topoisomerase mutations in clinical isolates of Morganella morganii harbouring the qnrD gene. Annals of Clinical Microbiology and Antimicrobials. 2014;13:34. doi: 10.1186/s12941-014-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power et al. (2006).Power P, Galleni M, Ayala JA, Gutkind G. Biochemical and molecular characterization of three new variants of AmpC β-Lactamases from Morganella morganii antimicrob. agents. Chemotherapy. 2006;50(3):962–967. doi: 10.1128/AAC.50.3.962-967.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat & Nair (2010).Rawat D, Nair D. Extended-spectrum β-lactamases in Gram Negative Bacteria. Journal of Global Infectious Diseases. 2010;2(3):263–274. doi: 10.4103/0974-777X.68531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliu, Vahjen & Zentek (2017).Saliu EM, Vahjen W, Zentek J. Types and prevalence of extended-spectrum beta-lactamase producing Enterobacteriaceae in poultry. Animal Health Research Reviews. 2017;18(1):46–57. doi: 10.1017/S1466252317000020. [DOI] [PubMed] [Google Scholar]
- Schultsz & Gerlings (2012).Schultsz C, Gerlings S. Plasmid mediated resistance in enterobacteriaceae; changing landscape and implications for therapy. Drugs. 2012;72(1):1–16. doi: 10.2165/11597960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Sedighi et al. (2017).Sedighi M, Halajzadeh M, Ramazanzadeh R, Amirmozafari N, Heidary M, Pirouss S. Molecular detection of β-lactamase and integron genes in clinical strains of Klebsiella pneumoniae by polymerase chain reaction. Revista da Sociedade Brasileira de Medicina Tropical. 2017;50(3):321–328. doi: 10.1590/0037-8682-0001-2017. [DOI] [PubMed] [Google Scholar]
- Seija et al. (2015).Seija VM, Presentado JC, Bado I, Papa Ezdra R, Batista N, Gutierrez C, Guirado M, Vidal M, Nin M, Vignoli R. Sepsis caused by New Delhi metallo-β-lactamase (blaNDM-1) and qnrD-producing Morganella morganii, treated successfully with fosfomycin and meropenem: case report and literature review. International Journal of Infectious Diseases. 2015;30:20–26. doi: 10.1016/j.ijid.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Şahinturk et al. (2016).Şahinturk P, Arslan E, Buyukcangaz E, Sonal S, Şen A, Ersoy F, Webber MA, Piddock LJV, Cengiz M. High level fluoroquinolone resistance in Escherichia coli isolated from animals in Turkey is due to multiple mechanisms. Turkish Journal of Veterinary And Animal Sciences. 2016;40:214–218. doi: 10.3906/vet-1506-74. [DOI] [Google Scholar]
- Shin et al. (2012).Shin G, You M, Lee H, Lee C. Catheter-related bacteremia caused by multidrug-resistant Leclercia adecarboxylata in a patient with breast cancer. Journal of Clinical Microbiology. 2012;50(9):3129–3132. doi: 10.1128/JCM.00948-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla et al. (2010).Singla N, Kaistha N, Gulati N, Chander J. Morganella morganii could be an important Intensive Care Unit pathogen. Indian Journal of Critical Care Medicine. 2010;14(3):154–155. doi: 10.4103/0972-5229.74176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó et al. (2018).Szabó O, Gulyás D, Szabó N, Kristóf K, Kocsis B, Szabó D. Plasmid-mediated quinolone resistance determinants in Enterobacteriaceae from urine clinical samples. Acta Microbiologica et Immunologica Hungarica. 2018;23:1–11. doi: 10.1556/030.65.2018.012. [DOI] [PubMed] [Google Scholar]
- Tamang et al. (2013).Tamang MD, Nam H, Gurung M, Jang G, Kim S, Jung S, Park YH, Lim SK. Molecular characterization of CTX-M-lactamase and associated addiction systems in Escherichia coli circulating among cattle, farm workers, and the farm environment. Applied and Environmental Microbiology. 2013;79(13):3898–3905. doi: 10.1128/AEM.00522-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh et al. (2005).Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo β-lactamases: the Quiet before the storm? Clinical Microbiology Reviews. 2005;18(2):306–325. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2009).Wang M, Jacoby GA, Mills DM, Hooper DC. SOS regulation of qnrB expression. Chemotherapy. 2009;53:1892–1897. doi: 10.1128/AAC.00132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2004).Wang M, Sahm DF, Jacoby GA, Hooper DC. Emerging plasmid-mediated quinolone resistance associated with the qnr gene in Klebsiella pneumoniae clinical isolates in the United States. Antimicrobial Agents and Chemotherapy. 2004;48:1295–1299. doi: 10.1128/AAC.48.4.1295-1299.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2016).Wu H, Wang M, Liu Y, Wang X, Wang Y, Lu J, Xu H. Characterization of antimicrobial resistance in Klebsiella species isolated from chicken broilers. International Journal of Food Microbiology. 2016;2(232):95–102. doi: 10.1016/j.ijfoodmicro.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Yuan et al. (2012).Yuan J, Xu X, Guo Q, Zhao X, Ye X, Guo Y, Wang M. Prevalence of the oqxAB gene complex in Klebsiella pneumoniae and Escherichia coli clinical isolates. Journal of Antimicrobial Chemotherapy. 2012;67(7):1655–1659. doi: 10.1093/jac/dks086. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2012).Zhao C, Tang N, Wu Y, Zhang Y, Wu Z, Li W, Qin X, Zhao J, Zhang G. First reported fatal Morganella morganii infections in chickens. Veterinary Microbiology. 2012;4; 156(3–4):452–455. doi: 10.1016/j.vetmic.2011.11.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.