Figure 2.
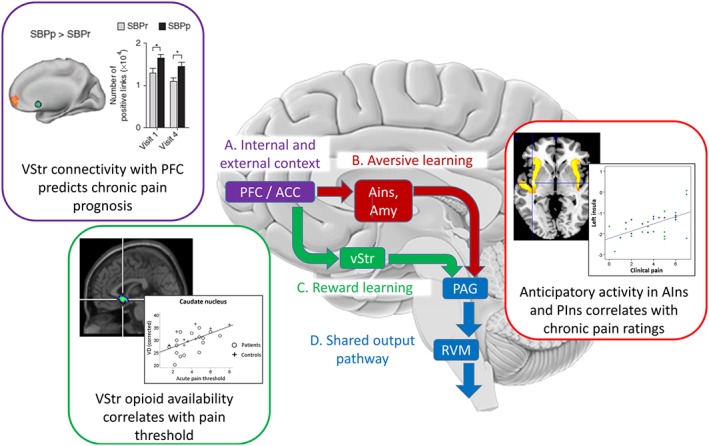
Opioidergic modulation of motivational learning processes play a role in the contextual modulation of pain. For illustrative purposes, the scheme simplifies the main opioid‐mediated, top‐down pathways involved in the modulation of nociception by internal (e.g. beliefs, mood and distress) and external (e.g. placebo treatment) context. Further bottom‐up and recurrent pathways exist but are not illustrated for clarity and were recently reviewed elsewhere (Büchel et al., 2014; Navratilova and Porreca, 2014). (A) Many cortical systems potentially mediate phenomena such as placebo hypoalgesia (Büchel et al., 2014), but all are likely to originate within the prefrontal cortex and its interactions with the perigenual Anterior Cingulate Cortex (ACC) as shown by their activation during anticipation of pain and prediction of placebo analgesia (Wager et al., 2004, 2011). These regions, through interactions with limbic areas, provide contextual cognitive information critical to the learning of reward and aversive value information (see Navratilova and Porreca, 2014). This is consistent with observations of abnormal Ventral Striatum (vStr) activity being a potential vulnerability factor in chronic pain (Purple inset, Baliki et al., 2012). SBPp: Subacute back pain, persistent. SBPr: Subacute back pain recovered. (B) Anterior Insula (AIns) and Amygdala (Amyg) form a circuit required for fear learning (Critchley et al., 2002) that shows opioid release during placebo analgesia (Zubieta et al., 2005). While anterior insula is deactivated during placebo analgesia (Price et al., 2008), it mediates the effects of negative expectations on increased pain (Brown et al., 2008b; Atlas et al., 2010) and shows abnormal anticipatory processing in patients with chronic pain (Red inset, Brown et al., 2014). (C) A role for opioidergic activity in vStr in setting nociceptive sensitivity/salience is evidenced by bidirectional modulation of vStr attenuating or enhancing nociception (Gear and Levine, 2011), which depends on upstream ACC opioid circuits (Navratilova et al., 2015). Consistent with this, pain thresholds in chronic pain patients correlate with opioid receptor availability in vStr (Green inset, Brown et al., 2015) presenting a potential vulnerability/resilience factor. (D) The cortical and subcortical projections all converge onto the PAG‐RVM‐spinal cord system which either inhibits or facilitates nociception (Fields, 2004).
