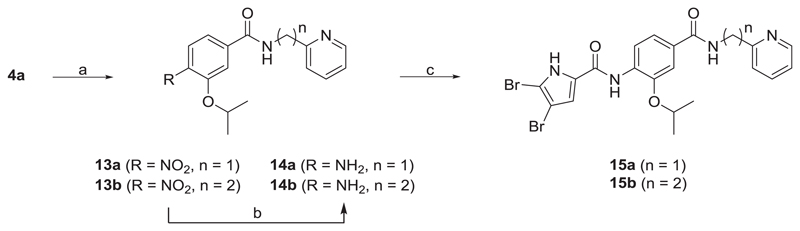
Scheme 3.
Reagents and conditions: (a) pyridin-2-ylmethanamine (for the synthesis of 13a) or 2-(pyridin-2-yl)ethan-1-amine (for the synthesis of 13b), TBTU, NMM, CH2Cl2, rt, 15 h; (b) H2, Pd-C, MeOH, rt, 5 h; (c) 4,5-dibromopyrrole-2-carboxylic acid, oxalyl chloride, CH2Cl2, rt, 15 h, then ii) 14a-b, pyridine, CH2Cl2, rt, 15 h.