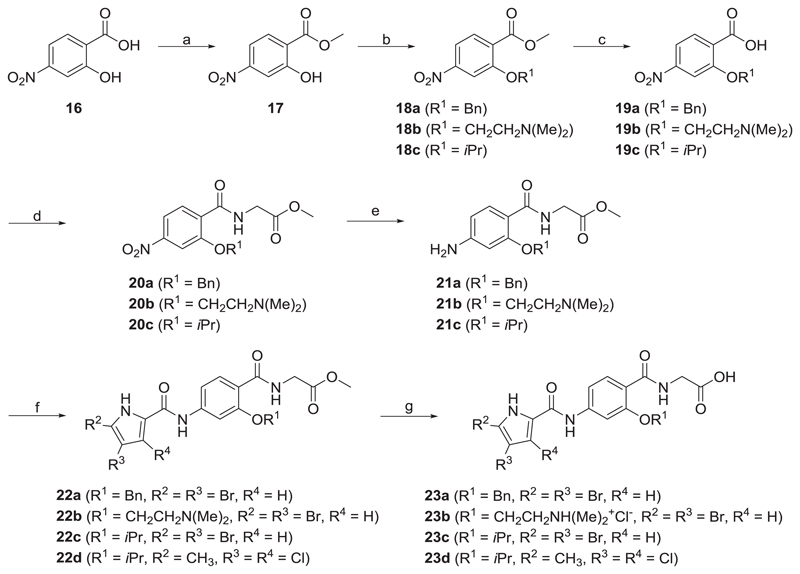
Scheme 4.
Reagents and conditions: (a) thionyl chloride, MeOH, 0 °C / rt, 15 h; (b) K2CO3, benzyl bromide, CH3CN, 60 °C, 15 h (for the synthesis of 18a), β-dimethyl-amino-ethylchloride hydrochloride, K2CO3, THF, 60 °C, 72 h (for the synthesis of 18b), isopropanol, triphenylphospine, DIAD, THF, rt, 15 h (for the synthesis of 18c); (c) 1 M NaOH, MeOH, rt, 15 h; (d) glycine methyl ester hydrochloride, TBTU, NMM, CH2Cl2, rt, 15 h; (e) SnCl2, EtOAc/MeOH, 55 °C, 15 h (for the synthesis of 21a), H2, Pd-C, MeOH, rt, 3 h, (for the synthesis of 21b-c); (f) the corresponding pyrrole carboxylic acid, oxalyl chloride, CH2Cl2, rt, 15 h, then ii) 21a-c, pyridine, CH2Cl2, rt, 15 h; (g) 1 M NaOH, MeOH/THF, rt, 15 h.