Table 1.
Inhibitory activity of type I compounds 7a-i, 8a-i, 9a-e, 10a-b, 11a-b, 12 and 15a-b against DNA gyrase from E. coli.
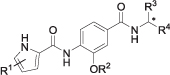 | ||||||
|---|---|---|---|---|---|---|
| Compd. | R1 | R2 | R3 | R4 | * | IC50 (nM)a or RA (%)b |
| E. coli gyrase | ||||||
| 7a | 4,5-diBr | iPr | iPr | COOMe | l | 94% |
| 7b | 4,5-diBr | iPr | Bn | COOMe | l | 100% |
| 7c | 4,5-diBr | iPr | CH3 | COOMe | l | 93% |
| 7d | 3,4-diCl-5-Me | iPr | Bn | COOMe | l | 100% |
| 7e | 3,4-diCl-5-Me | iPr | CH3 | COOMe | l | 66% |
| 7f | 3,4-diCl-5-Me | iPr | CH3 | COOMe | d | 87% |
| 7g | 4,5-diBr | CH2CH2NHBoc | H | COOMe | / | 53% |
| 7h | 4,5-diBr | CH2CH2NHBoc | Bn | COOMe | l | 100% |
| 7i | 3,4-diCl-5-Me | CH2CH2NHBoc | CH3 | COOMe | l | 100% |
| 8a | 4,5-diBr | iPr | iPr | COOH | l | 70% |
| 8b | 4,5-diBr | iPr | Bn | COOH | l | 100% |
| 8c | 4,5-diBr | iPr | CH3 | COOH | l | 370 ± 160 nM |
| 8d | 3,4-diCl-5-Me | iPr | Bn | COOH | l | 91% |
| 8e | 3,4-diCl-5-Me | iPr | CH3 | COOH | l | 38 ± 9 nM |
| 8f | 3,4-diCl-5-Me | iPr | CH3 | COOH | d | 41 ± 16 nM |
| 8g | 4,5-diBr | CH2CH2NHBoc | H | COOH | / | 96% |
| 8h | 4,5-diBr | CH2CH2NHBoc | Bn | COOH | l | 100% |
| 8i | 3,4-diCl-5-Me | CH2CH2NHBoc | CH3 | COOH | l | 590 ± 20 nM |
| 9a | 4,5-diBr | Bn | COOMe | l | 85% | |
| 9b | 3,4-diCl-5-Me | CH3 | COOMe | l | 34 ± 1 nM | |
| 9c | 4,5-diBr | H | COOH | l | 50% | |
| 9d | 4,5-diBr | Bn | COOH | l | 370 ± 10 nM | |
| 9e | 3,4-diCl-5-Me | CH3 | COOH | l | 28 ± 7 nM | |
| 10a | 3,4-diCl-5-Me | iPr | CH3 | CONHNH2 | l | 280 ± 80 nM |
| 10b | 3,4-diCl-5-Me | CH2CH2NHBoc | CH3 | CONHNH2 | l | 68% |
| 11a | 3,4-diCl-5-Me | iPr | CH3 | 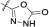 |
l | 85 ± 7 nM |
| 11b | 3,4-diCl-5-Me | CH2CH2NHBoc | CH3 | 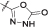 |
l | 1600 ± 200 nM |
| 12 | 3,4-diCl-5-Me | CH3 | 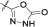 |
l | 13 ± 4 nM | |
| 15a | 4,5-diBr | iPr | H | 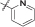 |
/ | 100% |
| 15b | 4,5-diBr | iPr | H | 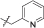 |
/ | 87% |
| novobiocin | 170 ± 20 nM | |||||
Concentration of compound that inhibits the enzyme activity by 50%.
Residual activity of the enzyme at 1 μM of the compound.
