Table 4.
2 h Biotransformation of sulfides and styrene. Conversions of 2 mM substrate were analyzed.
| Substrate | Product | VpStyA1 Conversion (%) | ee (%) | VpStyA2B Conversion (%) | ee (%) |
|---|---|---|---|---|---|
| PMS | 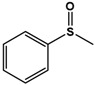 |
43.6 ± 1.9 | 98 (S) | 6.3 ± 0.3 | 64 (S) |
| 4F-PMS | 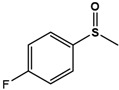 |
59.8 ± 0.4 | 99 (S) | 4.3 ± 0.7 | 84 (S) |
| 4Cl-PMS | 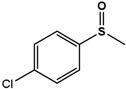 |
38.5 ± 1.7 | 99 (S) | 4.4 ± 0.4 | 96 (S) |
| 4Br-PMS | 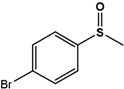 |
32.4 ± 4.7 | 99 (S) | 6 ± 0.7 | 96 (S) |
| BMS | 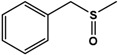 |
95 ± 0.5 | 97 (S) | 13 ± 0.9 | n.d. (S) |
| Styrene | 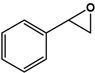 |
9.9 ± 0.4 | 98.2 (S) | 0.5 ± 0.1 | 45 (S) |
Phenyl methyl sulfide (PMS; thioanisole), 4-fluoro phenyl methyl sulfide (4F-PMS), 4-chloro phenyl methyl sulfide (4Cl-PMS), 4-bromo phenyl methyl sulfide (4Br-PMS), benzyl methyl sulfide (BMS); n.d. = not detectable.
