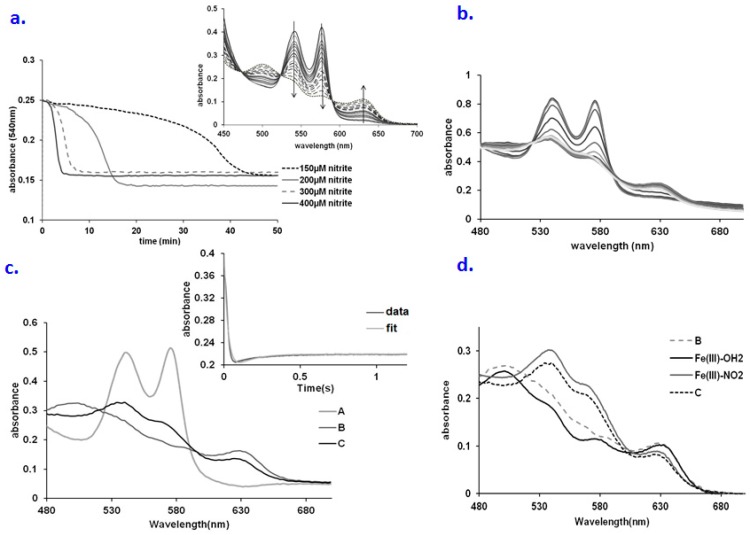
Figure 1.
(a) Time courses for the nitrite oxidation of hemoglobin in oxygen-saturated phosphate buffer saline (PBS) buffer at various concentrations of nitrite. Conditions: 25 µM Hb, PBS 7.4, room temperature; (b) UV-vis spectra collected upon mixing oxyHb (66.6 µM) with NaNO2 (660 mM). Conditions: pH 7.4, PBS buffer, aerobic, over a range of 2 s; (c) Computed spectra for the species involved in the A → B → C reaction model (A—oxyHb, C—metHb, D—met-nitriteHb). Conditions: 66.6 µM Hb, 0.66 M nitrite, pH 7.4, PBS buffer, aerobic. Inset: fitting at 575 nm trace for the A → B → C kinetic model; (d) Overlay of the computed spectra of species B and C with the spectra of various possible intermediates. Conditions: Fe(III)-OH2: oxyHb 30µM, pH 7.4; Fe(III)-NO2−: 30 µM metHb, 400 µM NaNO2, pH 7.4 PBS buffer; Fe(III)-OH2:oxyHb 30µM, pH 7.4 PBS buffer.