Table 1.
Yields for the two-step synthesis of 1-aryl-5-nitro-1H-indazoles.
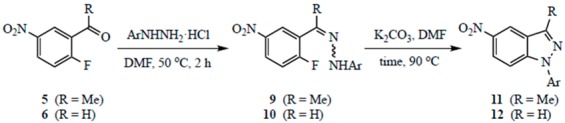
| Substrate | Ar | Time (h) a | Pdt | % Yield b |
|---|---|---|---|---|
| 5 | a Ph- | 2 | 11a | 95 |
| 5 | b 2-MeOPh- | 2 | 11b | 82 |
| 5 | c 3-MeOPh- | 2 | 11c | 81 |
| 5 | d 4-MeOPh- | 2 | 11d | 94 |
| 5 | e 4-BrPh- | 2 | 11e | 95 |
| 5 | f 3-ClPh- | 2 | 11f | 87 |
| 5 | g 4-ClPh- | 2 | 11g | 93 |
| 5 | h 2,4-Cl2Ph | 36 | 11h | 80 |
| 5 | I 3-CF3Ph- | 2 | 11i | 88 |
| 5 | j 4-CF3Ph- | 2 | 11j | 70 |
| 5 | k 4-CNPh- | 2 | 11k | 80 |
| 5 | l 4-H2NSO2Ph- | 10 | 11l | 70 |
| 5 | m 4-HO2CPh- | 24 | 11m | 75 |
| 6 | a Ph- | 2 | 12a | 72 |
| 6 | b 2-MeOPh- | 2 | 12b | 0 c |
| 6 | c 3-MeOPh- | 2 | 12c | 67 |
| 6 | d 4-MeOPh- | 2 | 12d | 70 |
| 6 | e 4-BrPh- | 2 | 12e | 70 |
| 6 | f 3-ClPh- | 2 | 12f | 60 |
| 6 | g 4-ClPh- | 2 | 12g | 70 |
| 6 | h 2,4-Cl2Ph- | 2 | 12h | 60 |
| 6 | I 3-CF3Ph- | 2 | 12i | 62 |
| 6 | j 4-CF3Ph- | 2 | 12j | 68 |
| 6 | k 4-CNPh- | 3 | 12k | 60 |
| 6 | l 4-H2NSO2Ph- | 2 | 12l | 50 |
| 6 | m 4-HO2CPh- | 2 | 12m | 50 |
a All hydrazones were generated in two hours. Times given are for the final cyclization. b Isolated yield of 1H-indazole for the two-step sequence. c Only the hydrazone was isolated.
