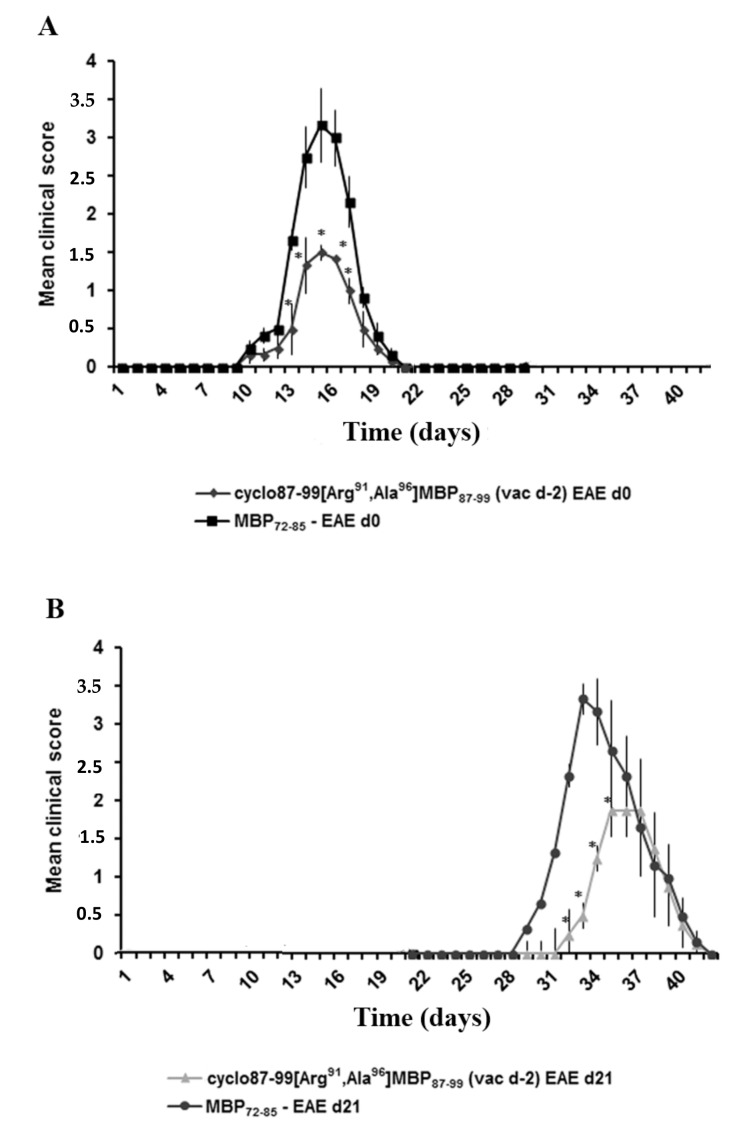
Figure 4.
Prophylactic administration of cyclic myelin basic protein (MBP) peptide analogue, cyclo(87–99)[Arg91, Ala96]MBP87–99, provides lasting protection against the development of MBP72–85-induced experimental autoimmune encephalomyelitis (EAE) in female Lewis rats. Mean clinical scores of MBP72–85-induced EAE in groups of rats that were treated prophylactically subcutaneously (s.c.) with cyclo(87–99)[Arg91, Ala96]MBP87–99 (500 μg), either 2 days, or 23 days before immunization for MBP72–85-EAE. A first group of untreated and treated rats (n = 6 per group) was immunized with MBP72–85 for the induction of EAE on day 2 relative to treatment with cyclic analogue (day 0 on graph) (A), and a second group (n = 4 per group) on day 23 relative to treatment with cyclic analogue (day 21 on graph) (B). Data are from one experiment. Statistical significance after pair-wise comparisons between groups of rats (using Mann–Whitney test) is shown (*, p < 0.05).