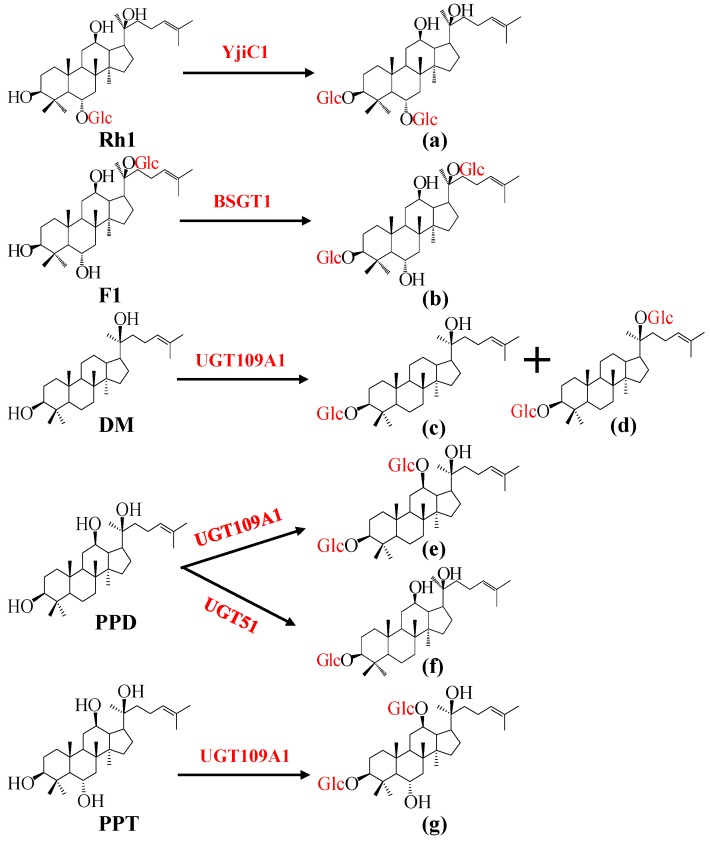
Figure 2.
Glycosylation of ginsenosides by UGTs from microorganisms. (a) 3-O-β-d-glucopyranosyl-6-O-β-d-glucopyranosyl-20(S)-protopanaxatriol; (b) (20S)-3β,6α,12β,20-tetrahydroxydammar-24-ene-20-O-β-d-glucopyranosyl-3-O-β-d-glucopyranoside (ginsenoside Ia); (c) 3-O-β-d-glucopyranosyl-dammar-24-ene-3β,20S-diol; (d) 3,20-Di-O-β-d-glucopyranosyl-dammar-24-ene-3β,20S-diol; (e) 3,12-Di-O-β-d-glucopyranosyl-dammar-24-ene-3β,12β,20S-triol; (f) ginsenoside Rh2; (g) 3,12-Di-O-β-d-glucopyranosyldammar-24-ene-3β,6α,12β,20S-tetraol.