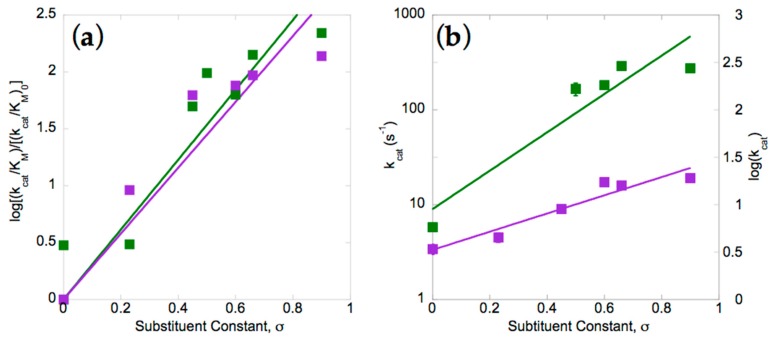
Figure 3.
Dependence of enzymatic reaction rates on para Hammett constant, for StNfsB (green) and MsPnbA (purple) [77]. (a) The second-order rate constant (kcat/KM) for each substituted substrate was divided by the value obtained for (unsubstituted) nitrobenzene before taking the log to get log[(kcat/KM)/(kcat/KM)0], which was plotted against the Hammett para substituent constant σ. Log[(kcat/KM)/(kcat/KM)0] plots yielded ρ values of 3.1 ± 0.2 (StNfsB, R2 = 0.86) and 2.9 ± 0.2 (MsPnbA, R2 = 0.83); (b) the first-order rate constants are plotted without normalization, yielding for StNfsB a slope of 2.4 ± 0.4 and an intercept of 0.9 ± 0.3 (R2 = 0.88), and for MsPnbA a slope of 1.0 ± 0.1 and intercept of 0.5 ± 0.08 (R2 = 0.92). See Methods for the equation and also Supplemental Table S2 and Figure S4 for compounds, and details.