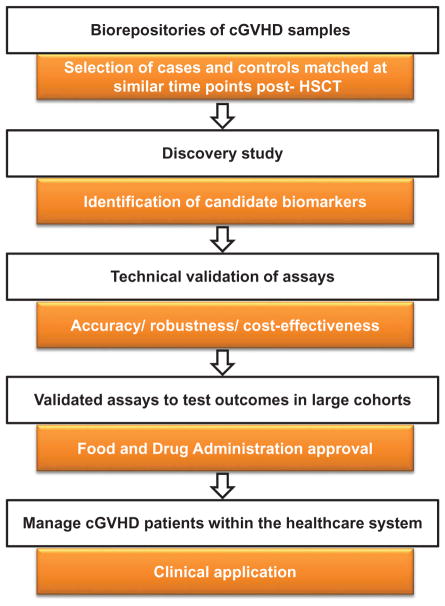
Figure 1.
Overview of cGVHD biomarkers workflow from discovery to validation studies.
Biorepositories of cGVHD patients are used to select cases and controls samples matched at similar time points post-HSCT for the discovery of candidate cGVHD biomarkers. Analytical assays are validated by assessing their accuracy, robustness and cost-effectiveness. Validated assays will move forward for validation in large cGVHD cohorts. Last, after approval from the Food and Drug Administration, tests are used in standard practice in cGVHD clinics.